Sandbox Reserved 465
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
KIF1A Motor Domain
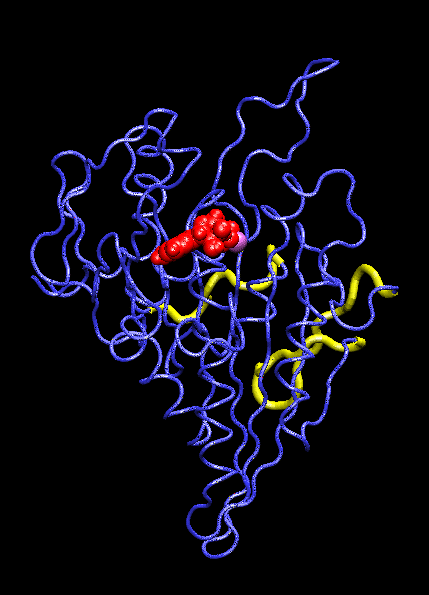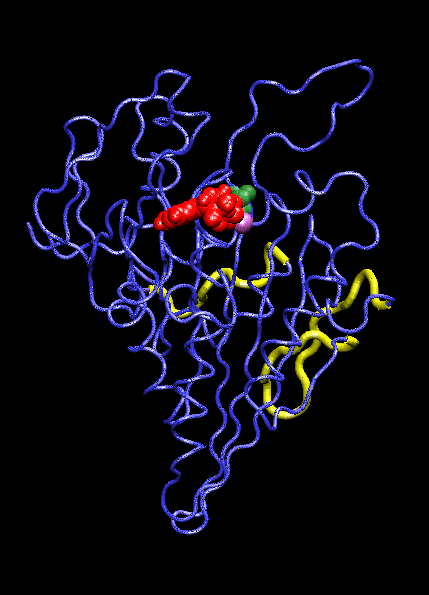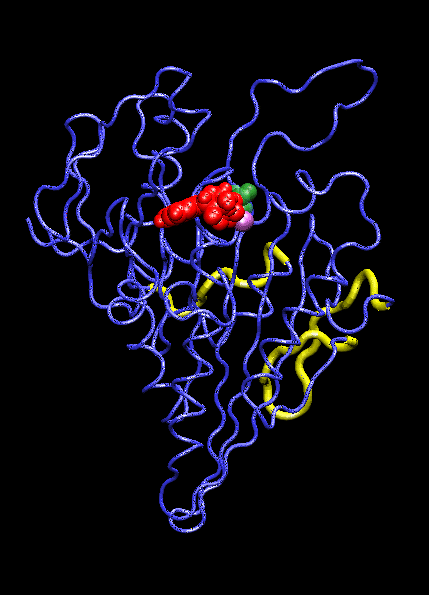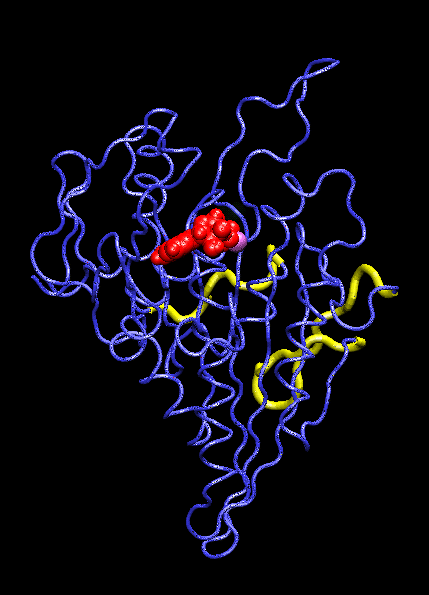
IntroductionThe KIF1A motor domain is a segment of the Kinesin-like protein KIF1A which is a motor protein. Kinesin is a microtubule motor that transports "cargo" by "walking" along a microtubule track. In KIF1A's case, it is a motor for anterograde axonal transport. The anterograde axonal transport is a cellular process that transfers synaptic vesicles from the neuron's cell body, through the cytoplasm of the axon towards the synapse. The kinesin protein is composed of a set of heavy chains that consist of the motor domain which leads to a stem. The stems connect at a light chain which is bonded to the cargo. So as the two motor domains "walk" the kinesin down the microtubule, the cargo is dragged behind. Kinesin mobility is made possible by hydrolyzing ATP which changes kinesin's structure and, in turn, generates movement down the track. For the majority, it is found in adult brain tissue (the cerebellum and cerebrum) within one specific type of neuronal cell. The KIF1A motor domain serves the same function wherever KIF1A is found, this includes humans and mice. Motor proteins(including their motor domains) are also utilized by types of fungi, plants, mammals, and insects. Defects with the KIF1A protein can result in spastic paraplegia, sensory neuropathy, and mental retardation. StructureThe KIF1A motor domain is a subunit of the KIF1A transport protein. The domain's secondary structures consist of a and eight . The motor domain uses as a ligand and energy source. The motor domain has two distinct conformation states, straight and bent. When saturated with ATP, a strong majority of the protein will be found in the bent state when bound to ADP. When the ligand is absent, the motor domain will be found in the straight formation. This bend occurs when the ATP binds and the energy from breaking the phosphorous-phosphorous bonds activates the relay helix which bends. This bend causes the whole protein to change confirmation, this change is referred to as the recovery stroke. This confirmation change bends the protein in a way that exposes the neck linker that was previously shielded by the rest of the protein. This exposed neck linker attaches itself to the track. As the neck linker bonds to the microtubule track, it continues to bond, creating a "zipping" mechanism. After the first motor domain binds to the microtubule, the ADP and inorganic phosphate detaches from the second motor domain which initiates another change in confirmation which straightens the protein. This straightening moves the domain forward while also concealing the neck linker. This straightening is referred is as recovery stroke. The KIF1A motor domain is mapped using a combination of x-ray crystallography and cryo-electron microscopy which analyzes force-generating conformation changes at atomic resolution.
Mechanism of ActionMolecular motors link the change of breaking ATP phosphate-phosphate bonds into structural change of the protein itself. When the, now, ADP is cleaved and the phosphate group dissociates, the ATP binding site is activated. This activation pushes on the relay helix which causes it to bend. When the helix bends it changes the overall protein and makes it capable of attaching to the microtubule track. After it attaches the inorganic phosphate and ADP are released from the complimentary domain. As this happens, the domain is pulled down the microtubule. AS this pull occurs, and the "power stroke" occurs and a pocket forms. This pocket, once again, exposes the neck linker which is able to "zip" into the protein, anchoring it to the track. This complete protein mechanism is referred to as kinesin walking. Medical Implications or Possible ApplicationDefects in these domains can inhibit kinesin movement. Without proper kinesin performance, transportation of crucial nutrients is inhibited and can lead to KIF1A defects including: spastic paraplegia autosomal recessive type 30 (SPG30), hereditary sensory neuropathy type 2C (HSN2C), and mental retardation autosomal dominant type 9 (MRD9). Spastic paraplegia autosomal recessive type 30, or SPG30, is a type of spastic paraplegia. Spastic paraplegia is a neurodegenerative disorder that is characterized by gradual weakness and lower back spasms. The severity of the symptoms and the rate of progression range from case to case. At first, balancing becomes difficult and stiffness and weakness occurs in the legs. This also leads to muscle spasms and dragging of the toes. The SPG30 spreads through the body, loss of bladder function and stiffness of other body parts can occur. Hereditary Sensory Neuropathy Type 2C, or HSN2C, is a neurodegenerative disorder that is characterized after a decade of progressive sensory loss in outer body parts, such as the fingers and toes. This sensory loss can lead to ulceration and amputation of these body parts. Also, muscle weakness in the legs and feet is common. Mental Retardation Autosomal Dominant Type 9, or MRD9, is characterized by below average intellectual function along with a lack of adaptive behavior.
Referenceshttp://www.rcsb.org/pdb/results/results.do?qrid=F4B2AD40&tabtoshow=Current http://www.uniprot.org/uniprot/Q12756 https://valelab.ucsf.edu/publications/2000casecurrbiol.pdf http://www.rcsb.org/pdb/explore.do?structureId=1I5S www.uniprot.org/uniprot/F5H045 http://www.rcsb.org/pdb/explore.do?structureId=1I5S https://valelab.ucsf.edu/publications/2000casecurrbiol.pdf
http://www.ncbi.nlm.nih.gov/pubmed/19966224 |