Sandbox Reserved 471
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
IntroductionActin is a spherical 43 kilo-Dalton protein that is found in many cell types. It belongs to the class of proteins known as motor proteins. Motor proteins function in the movement of substances across a substrate by utilizing ATP as an energy source. It is one of the most highly conserved proteins differing by no more than 20% in species ranging from yeast to humans.[1] In an alignment of the 81 unique actin protein sequences, scientists found that 17.4% of positions in the consensus sequence are invariant in all actins, 35.6 % of positions are invariant or have a difference in only one of the 81 actins, and 40.4% of positions are invariant or have what are considered conservative changes. [2] Actin is the monomeric subunit of microfilaments, one of the three key components of the cytoskeleton and thin filaments, which is part of the contractile unit in muscle cells. The cytoskeleton is a three-dimensional complex of filamentous protein that creates the shape and structure of cells, as well as fills in the gaps between organelles. This is what provides the cells with motility. There are three main proteins that make up the cytoskeleton. They are microfilaments, intermediate filaments and microtubules.[3] Actin exists in two forms, the monomeric, globular form (G-actin) and the polymeric, filamentous form (F-actin).[4] In vertebrates there are three main groups of actin isoforms which include alpha, beta, and gamma. These isotypes (α, β and γ), show >90% amino-acid homology between isotypes and >98% homology within members of a particular isotypic group.[5] The alpha actins, found in muscle tissues, are a major constituent of the contractile apparatus. The beta and gamma actins coexist in most cell types as components of the cytoskeleton, and as mediators of internal cell motility.
HistoryIn 1942, Bruno Ferenc Straub isolated actin as a water soluble component of muscle acetone powder. He found that at increased ionic strength the G-actin molecules would come together to form the F-actin filament. Actin was later found in non-muscle cells and its structure was seen through the use of immunofluorescence microscopy. The next major accomplishment came with the discovery of the amino acid sequence. This allowed researchers to locate chemical and enzymatic alterations and to study how they affected the properties of the molecule. The high conservation of actin’s structure throughout various species was discovered through comparative sequence analysis. In 1950, Straub reported that actin contained bound ATP and that during the polymerization of the monomers into microfilaments, the ATP was hydrolyzed to ADP and inorganic phosphate.[6] His discovery led to his suggestion that ATP-bound actin transforming into ADP-bound actin was what created muscle contractions. In the early 1980’s the principles of actin polymerization were exposed and the first actin-binding proteins (ABPs) were characterized according to their in vivo and in vitro functions. In 1990, the crystal structure of G-actin was solved by Kabsch.[7]In this same year a model of F-actin was suggested by Holmes et al. The model was created by fitting a helix of G-actin structures according to low-resolution fiber diffraction data from the F-actin.[8] Further advances in cryoelectron microscopy allowed scientists to find the binding sites for myosin and tropomyosin. A combination of X-ray crystallography with fiber diffraction led to an atomic model of F-actin.[9] After some debate actin also became generally accepted as an important structural and functional component of the cell nucleus [10] Since this pioneering work by Straub, interest in actin has increased tremendously and so has the realization of how complex its structure is.
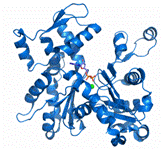
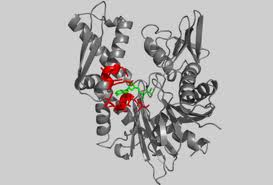
Structure
Actin exists in both its monomeric (globular, G-actin) and polymeric (filamentous or F-actin) form. The actin monomer is approximately pear shaped. Actin is composed of 376 residues that are folded into two large domains, each comprised of two subdomains. Its is 41% helical (22 helices; 153 residues) and 20% beta sheet (20 strands; 77 residues). The actin filament is a double-stranded, right-handed helix with a half-pitch of 37 nm and a one-start left-handed genetic helix with a rise of 2.75 nm per monomer. The width of the filament is within the range of 7–10 nm. [11] The large domains are organized to form a hinged molecule with a deep cleft. Subdomains 1 and 3 are structurally related, whereas subdomains 2 and 4 can be viewed as large insertions into subdomains 1 and 3, respectively. There is moderately little contact between the two major domains of actin; the polypeptide chain passes twice between these domains at the loop centered at residue Lys336 and at the linker helix Gln137-Ser145, which functions as the hinge axis between the domains. As a result, two clefts are formed between the domains. Within the upper cleft are the cofactors, an adenine nucleotide and a divalent metal ion, usually magnesium. It is thought that the cofactors interact with the domains on either side, increasing their connectivity. The lower cleft between domains 1 and 3 is lined by residues Tyr143, Ala144, Gly146, Thr148, Gly168, Ile341, Ile345, Leu346, Leu349, Thr351, and Met355, which are predominantly . The can also be seen, however they are not as involved in the binding site. This cleft constitutes the major binding site for most ABPs, and is thus called the targetbinding or hydrophobic cleft.Most of the interaction that occur in the subunit are electrostatic in character but there are also hydrophobic interactions.[12]
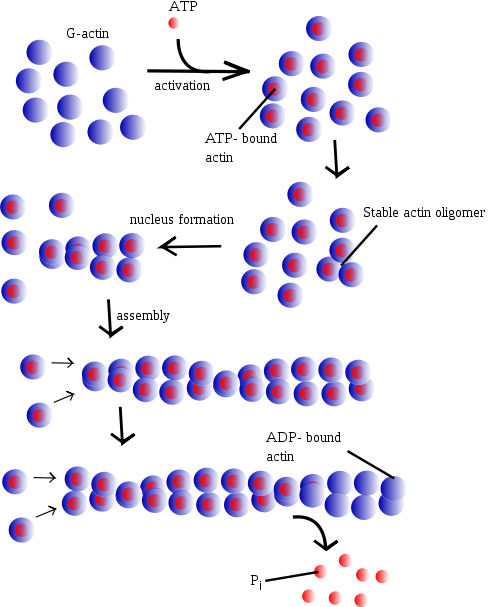
Under physiologic conditions, G-actin is transformed to F-actin by ATP. ATP is one of the most common binding in the cleft of actin. After G-actin is bound to an ATP molecule it can then bind to another ATP bound monomer to form an unstable dimer. It can then add a third ATP bound monomer to create a stable trimer that serves as a basis for building fibrous actin (F-actin). Once the F-actin is formed the ATP can be hydrolyzed, keeping the bound ADP and releasing an inorganic phosphate. The polymer can undergo a process known as treadmilling. This is where the polymer continuously grows at its positive end but disassembles at its negative end. This process requires a permanent source of energy such as ATP [13]
Unlike G-actin, F-actin does not form crystalline arrays that can be analyzed by X-ray crystallography. Instead it can be found by using fiber diffraction, electron-microscopy data and mathematical models. The structure can then be refined and remodeled as necessary. [14] The first high-resolution structural model of the actin filament was at a resolution of 8 A ˚ and was proposed by Holmes et al., 1990. Recently, an improved high-resolution F-actin model was created by Oda et al., 2009 with a resolution of 3.3 A ˚ in the radial and 5.6 A ˚ in the equatorial directions. Vertebrates express three main actin isoforms, including three α-isoforms of skeletal, cardiac, and smooth muscles and the β- and γ- isoforms expressed in nonmuscle and muscle cells. Actin isoforms are different in a few of its amino acids, with most variations occurring toward the N terminus. Actin also undergoes various forms of posttranslational modifications. For instance, His73 of skeletal muscle α-actin is methylated, the N-terminal methionine and cysteine residues are acetylated and cleaved, and the resulting N-terminal aspartic acid is then re-acetylated. Since the original determination of the crystal structure of G-actin, over 80 structures of actin have been reported. The majority of these structures have been obtained as complexes with actin-binding proteins (ABPs).[15]
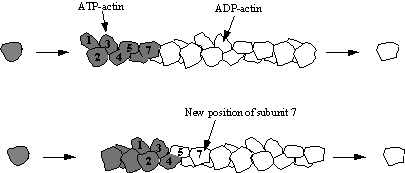
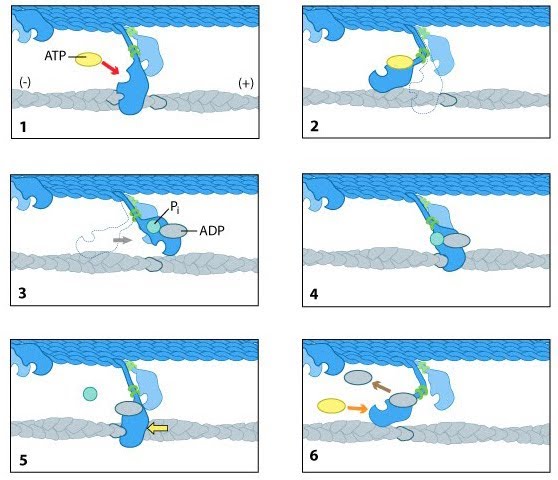
Mechanism of ActionPolymerization mechanismAt increased ionic strength, G-actin molecules polymerize into filaments, known as F-actin. F-actin is the main component of the thin filaments in sarcomeres of muscle cells. Muscle sarcomeres use ATP hydrolysis to produce conrtractions by sliding the thin actin filament past thick myosin filaments. The entire process is initiated by the slow formation of actin dimmers and trimers. These serve as a base for filament elongation. During elongation more actin monomers associate to than dissociate from either of the two ends, which results in the net growth of both filament ends. The steady-state phase is characterised by a dynamic equilibrium where the length of the actin filaments remains constant. This means while monomers are associating, other monomers are dissociating from the ends. In this dynamic equilibrium a stationery population of free actin monomers is established and is called the critical concentration. The polarity of the ends of the actin filaments play a key role in elongation. The ends of the filament are referred to as "barbed" and "pointed" according to the polarity of the arrowhead-like structure generated on binding with myosin subfragment 1.[16] The barbed or plus end binds actin monomers faster than the pointed or minus end. Although its ATPase activity is not crucial for actin polymerisation, actin self-assembly is associated with the ATPase cycle, which powers this treadmilling process. [17] After the actin molecule is changed into a filament, the bound ATP is hydrolysed to ADP and inorganic phosphate is realeased.[18] The formation of the F-actin is what stimulates the actin ATPase. The release of the inorganic phosphate happens more slowly than the formation of the filament so that the growing filament has a cap of ATPactin at its barbed end, while monomers containing ADP and inorganic phosphate accumulate in the rest of the filament.
Power Stroke MechanismMyosin II molecules bound to actin filaments generate force in skeletal muschles through a mechanism known as the power stroke. The mechanism is comprised of a cycle of ATP-binding, hydrolysis and phosphate release. At the beginning of the first step, the myosin head lacks an ATP and is attached to an actin filament in a conformation known as the “rigor confirmation.” In step two, the ATP binds to the myosin head which induces a conformational shift in the actin-binding site that reduces its binding to actin and cause myosin to release the actin. In the third step the binding of ATP leads to the myosin head being positioned further along the filament. The ATP is then hydrolysed and the inorganic phosphate and ADP remain bound to myosin. In the fourth step, the myosin head makes contact with the actin filament leading to another conformational change that promotes the release of inorganic phosphate. In step five, the binding between myosin and actin is reinforced and triggers the power stroke. Forces are generated on the actin filament as the myosin head returns to its original position. In the final step, the ADP is released and the myosin head remains bound to the filament at a new position from where it began therefore starting the cycle again.[21]
FunctionStudies of actin have shown that it has many functions within living cells. This range of functions is due to the diverse morphology of its structures and its interactions with actin-binding proteins (ABPs).[22] The diversity in the functional ability of actin proteins is due in part to its interaction with actin-binding proteins. F-actin and G-actin interact with a wide array of proteins. There are over 150 known actin binding proteins (ABPs), which makes up 25% of cellular protein. [23] Actin has proved to be an important component in the cytoskeleton. The actin cytoskeleton is known as the microfilament and has a multi-purpose role in important cell processes such as maintaining cellular polarity, cell shape, cell motility, adhesion, cytokinesis and endocytosis.[24] Myosin molecules are a class of actin motors that aid in creating movement by “walking” across actin filaments and contracting muscle tissues. Microfilaments, also known as thin filaments are helical polymers composed of globular actin (G-actin). The polymers are polar and bind to nucleotides such as ATP in order to hydrolyze them.[25] Actin has no important enzymatic activity, however, it inhibits DNase I and activates the myosin ATPase. Actin filaments activate the Mg2+ ATPase activity and the movement of myosin along actin filaments produces the force for muscle contraction and other cell movements. Motility mechanisms which involve actin are controlled by the interaction in the G or F form with the various ABPs. (Structure and Function of Actin by Wolfgang Kabsch) The mechanism is controlled by Ca2+ stimulated myosin light chain kinase. MLCK regulates the coming together of myosin and actin filaments and controls the force with which they contract. Actin-myosin filament networks are found in cells such as neurons and epithelial cells that don’t require movement. These networks provide neurons the ability to stretch cell processes long distances. In higher order eukaryotic cells, actin filaments and myosin II filaments often come together to perform a function and then disassemble. For example in cytokineses, actin and myosin II filaments organize to form a contractile ring. The ring exists beneath the plasma membrane and during the M-phase of cell division, the ring contracts, pulling the plasma membrane inward and constricts the middle of the cell, leading to an eventual separation into two daughter cells. [26]
Medical ImplicationsMany of the functions of the cytoskeleton involve cytoskeleton and plasma membrane interactions. Proteins that exist between the two help to control cell shape, define the membrane domains and regulate cell/cell interactions and adhesion. There are at least 15 major protein species involved in the membrane-cytoskeleton of the human red blood cell. A mutation in any of these proteins can lead to cell fragility and cell death. Homologs of the erythrocyte membrane-skeleton can be found in many other cell types, which suggests the significance of these proteins. One of particular importance is the actin associated protein dystrophin. Dystrophin is an important part of the sarcolemmal membrane where it links the membrane to the sides of the actin filament bundles. Mutations in this protein leads to muscular dystrophies such as Duchenne’s disease or Becker muscular dystrophy. A homolog of dystrophin called utropin is thought to play a similar role in non-muscle cells. [27] The genome of many mammals, including humans contains six actin genes, ACTA1, ACTA2, ACTB, ACTC, ACTG1 and ACTG2. Four of these are differentially expressed in cardiac (ACTC), smooth (ACTA2) enteric (ACTG2) and skeletal muscles (ACTA1); two are described as cytoplasmic actin genes (ACTB and ACTG1) and are expressed in all cells. [28] Actin mutations were not really known until the last 6-10 years. Mutations that have been found to cause human diseases have been found in the ACTC cardiac muscle gene which can cause dilated or hypertrophic cardiomyopathies including congenital fiber-type disproportion (CFTDP). Recently, mutations in ACTG1 have been associated with autosomal dominant deafness. Majority of actin disease mutations have been found to be dominant mutations. This has been the case for actin mutations is the Drosophila flight muscle-specific actin gene ACT88F. Scientists believe the common dominance of actin mutations is because the major functions of actin occur when it is in its F-form, associated with other proteins. The dominant actin mutations that lead to disease are mostly missense mutations. Actin mutations have been found in a variety of species such as yeast, nematodes and flies whose genomes are more accessible to mutagenesis. It is thought that there are three reasons why actin mutations are likely to be at very low frequencies in human populations: 1. actin is a ubiquitously expressed protein, 2. it is a highly conserved protein with many binding partners, so mutations in a large fraction of actin residues appear to cause a severe phenotype in humans and other organisms and 3. most actin mutations are dominant and given the usually severe effects, are not passed on to offspring. Thus familial actin mutants are likely to be relatively mild dominant alleles or recessive. Severe dominant alleles are likely to be de novo mutations. In the case of cytoplasmic actin, five ACT1G disease-causing mutant alleles are known so far and no disease-causing ACTB alleles have yet been described. References
[29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] [40] [41] |