This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 473
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
KinesinIntroductionKinesin is a superfamily of motor proteins, one of three major known groups of molecular motors. The other two groups are myosin and dynein. These molecular motors convert chemical energy into mechanical energy through ATP hydrolysis for transport within the cell. Kinesin is known for its directed “walking” motion along microtubules, which are key components of the cell cytoskeleton. Kinesin is believed to transport large cargo such as organelles, vesicles, and even chromosomes during mitosis and meiosis in most or all eukaryotic organisms [1].
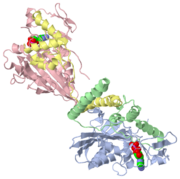
StructureThe structure of several kinesins have been elucidated primarily by X-ray crystallography, and also partly by electron microscopy [2]. The structure of conventional kinesin, a particular type of kinesin so named because it has been the most studied, is displayed in both the 2D and 3D figures shown. Conventional kinesin is a homodimer composed of two subunits, each with a heavy-chain (KHC) and light-chain (KLC) domain [1]. Together, these units form a protein composed of three main regions: the head, the stalk, and the tail. The tail is responsible for binding the cargo. The stalk usually consists of a structure similar to a coiled coil that helps to secure the two monomers together. (The tail and stalk are not shown in the figures.) The head, also known as the motor domain or the heavy chain, is responsible for microtubule binding and catalytic activity. It is composed of several and . The motor domain is the most conserved element among all kinesins, with 35% sequence homology across all kinesins. The stalk and tail share little homology, suggesting the diversity of cargo that different kinesins bind [3]. Key features within the motor domain include the , the , and the . The ATP-binding site consists of a P-loop motif common not only to kinesin, but also to myosin as well as several G-proteins which also have nucleotidase activity. This homology suggests a common ancestor among these proteins [3].
MechanismMolecular motors convert chemical energy to mechanical energy by causing reactions that power directed conformational changes, resulting in motion. In solution or in a free state, kinesin has bound to each head or motor domain. Once kinesin attaches to a microtubule and changes its conformation slightly, ADP can be exchanged for high-energy ATP. This action causes the to “zipper” into a binding pocket in the motor domain (near a structure called the ). The zippering causes the second head of the kinesin to swing around and land on the microtubule ahead of the first head [4]. The second head may reach the microtubule either by active movement, by simple diffusion, or by a combination of the two [3]. While the second head binds the microtubule, ATP hydrolysis occurs at the first head, releasing it from the microtubule. ATP hydrolysis also “unzippers” the neck linker from the relay helix, resetting the conformation for another reaction. It is remarkable that ATP binding and hydrolysis create tiny conformational changes in the active site (on the order of angstroms) that can lead to large overall conformational changes in the entire protein (on the order of nanometers). This phenomenon is known as mechanical amplification [5]. The reaction repeats over and over again as the kinesin binds and hydrolyzes one ATP for each 8-nanometer step it takes [6]. Once bound to the microtubule, kinesin can take several steps without falling off, an ability known as processivity.
DirectionalityKinesins walk along microtubules, which are a key component of the cytoskeleton and are composed of heterodimers of α- and β-tubulin. A microtubule is in the shape of a hollow cylinder with a circumference of 13 tubulin units [4]. The heterodimers layer in a helical fashion, giving the microtubule polarity. The ends are labeled as plus and minus, with the minus-end typically anchored near the center of the cell at a centrosome, and the plus-ends branching out towards the cell membrane [1]. This polarity is critical for the directed movement of kinesin because it allows the kinesin to recognize the directionality of the microtubule and transport its cargo in the right direction. Kinesins travel in only one direction along the microtubule. Typically, they travel from the minus end to the plus end, but some kinesins (such as ncd) travel in the opposite direction [3].
RegulationRegulation of kinesin is not fully understood, but it is believed to occur at the cargo-binding tail or light chain (KLC) domain of the protein [3].
ApplicationsWhile kinesins are generally studied for understanding of structure, mechanism and function, kinesin might be implicated in several diseases in which defective transport occurs in cells. For instance, kinesin may be involved in causing the protein aggregation that leads to neurodegenerative diseases such as Alzheimer's. Better understanding of the mechanism of kinesin, and if and how it is responsible in causing these diseases, could lead to new treatments. Additionally, since kinesin is critically involved in cell replication through mitosis, it could be targeted in cancer treatments as a way to prevent cancer cell division [7]. References
|