To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
For more help, look at this link:
http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
ACETYLCHOLINE RECEPTOR
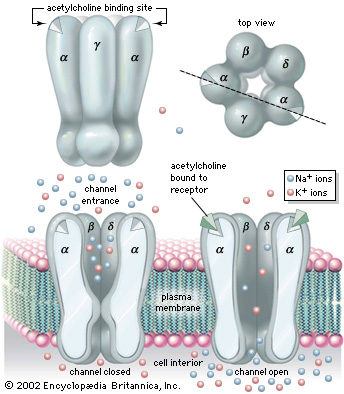
Nicotinic Acetylcholine Receptor (nAChRs) is a neurotransmitter receptor also known as a specialized integral membrane protein. Integral membrane proteins are permanently bound to the phospholipid bilayer. They provide a communication pathway between the internal and external environments of the cell. Acetylcholine receptor proteins reside as ligand-gated ion channels on the motor end plate of the postsynaptic cleft in the neuromuscular junction.[1]
nAChRs as an ionotropic receptor, which are in direct facilitation of ion movement.[2] When a ligand binds to the , the channel responds by opening. Likewise, when the ligand is removed the channel closes. They are activated by the binding of the acetylcholine neurotransmitter. Once the receptor is activated the ion channel opens allowing the exchange of salt ions. Nicotinic acetylcholine receptors are responsible for the regulation of sodium (Na+) ion concentration entering and potassium (K+) ion concentration leaving the muscular cytosol, ultimately resulting in an action potential from the differences in electrochemical gradients.[3]
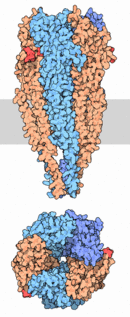
Acetylcholine receptor proteins are present in the peripheral nervous system (PNS) as well as the central nervous system (CNS) in humans and many organisms, including invertebrates. The function of the receptor follows a general mechanism to which it is applied to almost any organism with a nervous system, that relies on the firing action potentials of the neurons.[4] The structure depicted to the right is a redering of the the nicotinic acetylcholine receptor extracted from Torpedo marmorata, more commonly known as the Marbled Electric Ray. Specifically, it is extracted from the electric organ's tissue from within the organism.[5]
Structure
Nicotinic Acetylcholine Receptor is composed up five distinct subunits (shown in figure to left): α1, α2, β1, δ, and ε at approximately a molecular mass of 290 kDa. The subunits are symmetrically arranged around a central pore. Each subunit is a transmembrane domain with the and located on the extracellular side of the plasma membrane. Each subunit are composed of long protein chains that extend across the cell membrane. The subunits have a composition of around ~20% and ~40% from about ~370 residues in total.[5]
In vertebrates, nicotinic receptors are classified by their main sites of expression into two subtypes: muscle-type nicotinic receptors and neuronal-type nicotinic receptors. In the muscle-type receptors, found at the neuromuscular junction, receptors are either the embryonic form, composed of α1, β1, δ, and γ subunits in a 2:1:1:1 ratio, or the adult form composed of α1, β1, δ, and ε subunits in a 2:1:1:1 ratio. The neuronal subtypes are various combinations of twelve different nicotinic receptor subunits: α2 through α10 and β2 through β4. In both muscle-type and neuronal-type receptors, the subunits are somewhat similar to one another, especially in the hydrophobic regions.
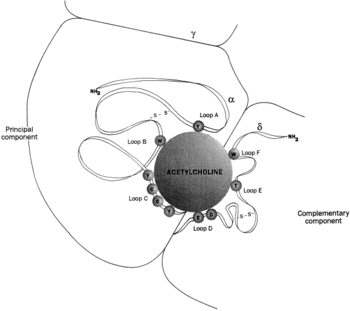 As shown in the picture to the right, rendered using electron microscopy to show the two α-subunits are colored orange in this homeric receptor example. The two α-subunits each contain a ligand binding site colored red. Cys-192 and Cys-193, which are close proximity to the allosteric binding sites on the α-subunits for acetylcholine have been experimentally tested to show that disulfide residues provides stability in the binding of the neurotransmitter.[5] The image (bottom left[6]) shows a simplified depiction of the main components of one of the binding sites in a nAChR. Acetylcholine is shown to interact with various amino acid residues but specifically with the series of amino acids: Tyrosine (Y), Cysteine (C), Cysteine (C), and Tyrosine (Y). The cysteine sulfide residues form the disulfide interaction which aids in acetylcholine binding. The disulfide bonds facilitate the loop formations necessary for the appropriate bonds being formed with acetylcholine.[7]
Mechanism
The neuromuscular junction is the location where the neuron activates muscle to contract. This is a step in the excitation-contraction coupling of skeletal muscle.
- Action potential travels down the axon of the activated presynaptic neuron.
- Upon the arrival of an action potential at the presynaptic neuron terminal, voltage-gated calcium channels open and Ca2+ ions flow from the extracellular fluid into the presynaptic neuron's cytosol.
- This influx of Ca2+ causes neurotransmitter-containing vesicles to dock and fuse to the presynaptic neuron's cell membrane through SNARE proteins.
- Fusion of the vesicular membrane with the presynaptic cell membrane results in the emptying of the vesicle's contents (acetylcholine) into the synaptic cleft, a process known as exocytosis.
- Acetylcholine diffuses into the synaptic cleft and binds to the nicotinic acetylcholine receptors bound to the motor end plate.
- These receptors are ligand-gated ion channels, and when they bind acetylcholine at the two specific subunits, it causes a conformational change to open and form the pore-like structure for the ion exchange to occur.
- Because of the differences in electrochemical gradients across the plasma membrane, more sodium moves in than potassium out, producing a local depolarization of the motor end plate known as an end-plate potential.
- This depolarization spreads across the surface of the muscle fiber and continues the excitation-contraction coupling to contract the muscle.
- The action of acetylcholine is terminated when the enzyme acetylcholinesterase degrades part of the neurotransmitter (producing choline and an acetate group) and the rest of it diffuses away.
- The choline produced by the action of acetylcholinesterase is recycled — it is transported, through reuptake, back into the presynaptic terminal, where it is used to synthesize new acetylcholine molecules.[8]
Medical Applications
Drugs and Toxins
nAChRs are the target sites of by many toxins such as those present in snake venom such as α-bungarotoxin.[9] These receptors can also be blocked by curare, hexamethonium as well as neuromuscular blocking agents such as those used in medical anesthesia. Neuromuscular blocking agents bind reversibly to nicotinic receptors in the neuromuscular junction.
A popular neurotoxin approved by the FDA was Botox Cosmetic which was used as a treatment for moderate to severe glabellar lines. Botox acts very similarly to the neuromuscular blocking agents in that it binds at the postsynaptic cleft to the receptors of the motor end plate and relaxes the two major muscle contractions in the forehead. Botox Cosmetic (botulinum toxin type A) blocks neuromuscular transmission by binding to acceptor sites on motor nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine, resulting in paralysis.[10] This inhibition occurs as the neurotoxin cleaves SNAP-25, a protein integral to the successful docking and release of acetylcholine from vesicles situated within nerve endings.
Diseases
Myasthenia gravis[11] is a disease where the body's antibodies actually target the acetylcholine receptors at the motor end plate and prevents correct functionality leading to muscular weakness. In a similar case, Lambert–Eaton myasthenic syndrome[12], is another rare autoimmune disease where the body's antibodies target the voltage-gated calcium channels at the presynaptic site and prevent electric signals from reaching the muscles that are in conjunction with the neuron.
References
- ↑ Purves, Dale, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience (4th ed.). Sinauer Associates. pp. 122–6. ISBN 978-0-87893-697-7.
- ↑ Connolly CN, Wafford KA (2004). "The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function". Biochem. Soc. Trans. 32 (Pt3): 529–34. doi:10.1042/BST0320529. PMID 15157178.
- ↑ Itier V, Bertrand D (August 2001). "Neuronal nicotinic receptors: from protein structure to function". FEBS letters 504 (3): 118–25. doi:10.1016/S0014-5793(01)02702-8. PMID 11532443.
- ↑ Guzman, F. Acetylcholine receptors: muscarinic and nicotinic. http://pharmacologycorner.com/acetylcholine-receptors-muscarinic-and-nicotinic/#what
- ↑ 5.0 5.1 5.2 Unwin N. (March 4, 2005). "Refined structure of the nicotinic acetylcholine receptor at 4A resolution". Journal of Molecular Biology 346 (4): 967–89. doi:10.1016/j.jmb.2004.12.031. PMID 15701510.
- ↑ Arias, H. (1998). Topology of ligand binding sites on the nicotinic acetylcholine receptor. 25:2, 133-191. www.sciencedirect.com/science/article/pii/S0165017397000209
- ↑ Rae, P., Karlin, A. (1986). Acetylcholine Receptor BindingSite Contains a Disulfide Cross-link between Adjacent Half-Cystinyl Residues*. 261:18, 8085-8088. www.jbc.org/content/261/18/8085.full.pdf
- ↑ Dale Purves, George J. Augustine, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and Leonard E. White (2008). Neuroscience. 4th ed.. Sinauer Associates. pp. 121–2. ISBN 978-0-87893-697-7.
- ↑ Samson, A. O.; Levitt, M. (2008). "Inhibition Mechanism of the Acetylcholine Receptor by α-Neurotoxins as Revealed by Normal-Mode Dynamics". Biochemistry 47 (13): 4065–4070.
- ↑ Botox Cosmetic (botulinum toxin type A) http://www.medilexicon.com/drugs/botox_cosmetic.php
- ↑ Conti-Fine BM, Milani M, Kaminski HJ (2006). "Myasthenia gravis: past, present, and future". J. Clin. Invest. 116 (11): 2843–54. doi:10.1172/JCI29894. PMC 1626141. PMID 17080188.
- ↑ Mareska M, Gutmann L (June 2004). "Lambert-Eaton myasthenic syndrome". Semin. Neurol. 24 (2): 149–53. doi:10.1055/s-2004-830900. PMID 15257511.
|