Sandbox Reserved 477
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Glyceraldehyde-3-Phosphate DehydrogenaseIntroductionGlyceraldehyde-3-Phosphate dehydrogenase is included in a class of proteins known as oxidoreductases. Enzymes classified as oxidoreductases are involved in redox reactions, catalyzing the transfer of electrons from one molecule to another. "Glyceraldehyde, commonly abbreviated as GAPDH, is approximately 37 kDa depending on the organism that particular enzyme was isolated from and is responsible for catalyzing the sixth step of a process called glycolysis, which serves to break down glucose for energy and carbon molecules for aerobic organisms. GAPDHs are cytoplasmic glycolytic enzymes, which although lacking identifiable secretion signals, have also been localized to the surface of several bacteria (and some eukaryotic organisms); where in some cases they have been shown to contribute to the colonization and invasion of host tissues." [1] GAPDHs are designated as class I and II GAPDHs. "Class I GAPDH is the classic enzyme of glycolysis and the Calvin Cycle in eubacteria and eukaryotes, while class II GAPDH has so far only been found in archaebacteria. In addition to the metabolic function, GAPDH has recently been implicated in several nonmetabolic processes, including transcription activation, initiation of programmed cell death, and ER to Golgi vesicle shuttling. GAPDH is considered a housekeeping gene due to its stability and expression at high levels in most tissues and cells. Housekeeping genes are genes that are required for the maintenance of basic cellular function, and are expressed in all cells of an organism." [2] For this reason, GAPDH is commonly used by researchers as a loading control for the Western Blot and as a control for Real Time PCR.
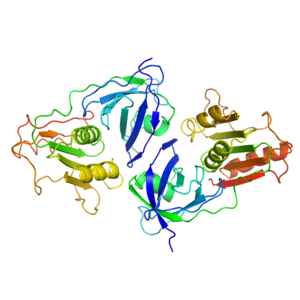
Structure"X- ray crystallography used to determine the crystal structure of the enzyme at 2.60 Å for the resolution." [3] Glyceraldehyde-3-Phosphate Dehydrogenase is a homotetramer and has chains of exactly the same sequence and structure. "Depending on the isoform of GAPDH, there are different numbers of secondary structures; however, the more common GAPDHs have 14 and 13 . Evolutionary change for GAPDH is very slow, so there is high sequence similarity between bacterial and eukaryotic GAPDHs. Though not shown, the residue Cysteine 139 is the active site at the C end of the alpha helix 138-153. In bacteria and eukaryotes, a His 176 is conserved. The histidine is believed to act as a base that extracts a proton from the Cys 139 during catalysis." [4] NAD+ is the most common for the binding of GAPDH. A ligand is a molecule that binds to a central atom to form a complex. NAD+ expresses negative cooperativity in relation to GAPDH; as NAD+ binds to the protein, the protein's affinity for the ligand decreases. Due this enzyme's high content of , GAPDH maintains efficient stability by numerous hydrophobic interactions within the molecule. DomainsGlyceraldehyde-3-Phosphate Dehydrogenase has two conserved domains in most isoforms. "The NAD Binding Domain is an Alpha Beta 3-layer sandwich with a central beta sheet covered on both sides of the alpha helices. It includes amino acids 1-138 and 301-340 on chains O and Q. The second domain is composed of extensive antiparallel beta sheet regions. This domain also contains an S-shaped loop of polypeptide residues 178-201, which are in contact with NAD+ on the R axis. The S-loop also interacts with several amino acids across the P axis. The S-loop forms the core of the four subunits, and most of the residues are internal and essential for the interaction across the R axis. The Q-, R-, and P-axes make up the three orthogonal twofold symmetry axes of GAPDH, which relate the four subunits together." [5]
DiseasesGlyceraldehyde-3-Phosphate Dehydrogenase deficiency is a rare genetic disorder in which an individual has a deficiency of GAPDH, which is heavily involved in breaking down carbohydrates consumed in the diet in order to produce energy. This condition is asymptomatic and affects less than 200,000 people in the United States. "GAPDH deficiency also occurs in plants, known as plastidial GAPDH deficiency. In plants, glycolysis occurs in both the cytosol and plastids. In a chloroplast/plastid-localized GAPDH isoform, gapcp, these double mutants have produced drastic phenotypes of arrested root development, dwarfism, and sterility." [6] GAPDH is a critical enzyme for all organisms. Major mutations in this enzyme could lead to almost immediate death of the cell. Nevertheless, GAPDH is not just a glycolytic protein. It is a multidimensional protein with nuclear, cytoplasmic and membrane functions. GAPDH may be involved in apoptosis and age-related neuronal diseases, such as Alzheimers; a subcellular reduction in GAPDH glycolytic activity (ie intracellular differences) is found in Alzheimer's and Huntington's disease cells. GAPDH is involved in the molecular mechanisms that are responsible for pathogenesis in the CAG trinucleotide repeat diseases. Eight inherited neurodegenerative diseases are known to be caused by expansion of the CAG repeat. It is possible that GAPDH's interaction with mutant proteins may damage brain neurons.
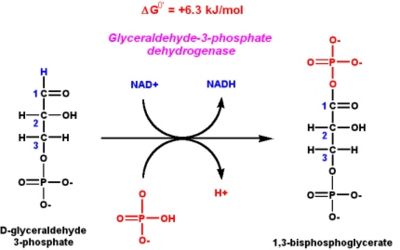
Mechanism of ActionThe mechanism using GAPDH to convert glyceraldehyde-3-phosphate into 1,3-bisphosphoglycerate in the sixth step of glycolysis is a two step process. The first reaction is the oxidation of glyceraldehyde-3-phosphate at the 1st carbon position. At this position, an aGAPDH Inhibitorsldehyde is converted into a carboxylic acid while NAD+ is simultaneously reduced to NADH. This reaction is energetically favorable with a Gibb's free energy of -50kJ/mol. The energy released by the first reaction drives the second reaction. GAPDH uses covalent catalysis and general base catalysis to decrease the very large and positive activation energy of the second step of this reaction. First, a cysteine residue in the active site of GAPDH attacks the carbonyl group of GAP, creating a hemithioacetal intermediate. Next, an adjacent, tightly bound molecule of NAD+ accepts a hydride ion from GAP, forming NADH; GAP is oxidized to a thioester intermediate using a molecule of water. Donation of the hydride ion by the hemithioacetal is facilitated by its deprotonation by a histidine residue in the enzyme's active site. Deprotonation encourages the reformation of the carbonyl group in the thioester intermediate and ejection of the hydride ion. NADH leaves the active site and is replaced by another molecule of NAD+. A molecule of inorganic phosphate is transferred to the intermediate to form the final product of this reaction that has a high phosphoryl-transfer reaction, 1,3-bisphosphoglycerate. • The normal dehydrogenase reaction involves NAD as coenzyme, phosphate and D-glyceraldehyde-3-phosphate as substrates. GAPDH Inhibitors
References
|