Sandbox Reserved 486
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
Diphtheria toxinDiphtheria Toxin is an exotoxin produced by the organism Corynebcterium diphtheria which has been infected by a bacteriophage that contains the Diphtheria toxin gene. The toxin is the causative agent of diphtheria. Symptoms for Diphtheria can range from a sore throat with low-grade fever and an adherent pseudomembrane of the tonsils, pharynx, or nose to infected skin lesions which lack a characteristic appearance.[1] The toxin is distributed to distant organs by the circulatory system and may cause paralysis and congestive heart failure.[2] The toxin attacks and kills eukaryotic cells by inactivating the Elongation factor (EF-2) in translation. EF-2 allows for translocation of the peptidyl-tRNA from the A-site to the P-site, which in turn frees the A site for another aminoacyl-tRNA to bind. By inactivating the elongation factor 2 during translation the protein being made cannot be completed and therefore becomes nonfunctional. The toxin is in a class of A-B which includes cholera toxin, Escherichia coli heat labile enterotoxin, Pseudomonas aeruginosa exotoxin A, tetanus, botulinum neurotoxins, and Shiga toxin. A-B class is characterized by two functionally distinct components such as component A is the catalytic components while component B does the receptor binding function.
HistoryScientist have made efforts since at least the 1740's to find the caustion and cure for diphtheria toxin. It wasn't until Koch's devolpment of medical microbiology and Koch's postulates that it was even possible for progression towards understanding the molecular causes. It was later in Koch's laboratory that Friedrich Loeffler isolated a bacteria from a patient that died from Diphtheria toxin. Koch and Friedrich later inoculated 23 guinea pigs with the isolated bacteria and they all died in two to five days. Loeffler had another observation that only the isolated bacteria from the pigs were growing at the site of inoculation. Therefore, he came to the conclusion that the bacteria isolated was the causation of the Diphtheria toxin. In Pasture institute in Paris, both Emile Roux and Alexandre Yersin were able to isolate the toxin by growing a pure culture of Diphtheria bacilli and forcing them through a porcelain filter. This allowed to obtain no bacteria and only the toxin. The toxin was injected into laboratory animals and caused the same symptoms of Diphtheria bacilli. It wasn't until sixty-five years later that protein crystals were produced and the structure obtained. Emil von Behring published a paper with the discovery of diphtheria antitoxin one week after Koch found out the resistance gained by vaccination from Diphtheria bacilli. This lead to elimination of the disease diphtheria in developing countries.
StructureDiphtheria toxin is a protein made of 535 amino acids and is 58kDa in weight. The proenzyme(zymogen) must be cleaved into fragments A & B and reduced in order for the toxicity gene to be turn on. In each fragment there are two . There are also three domains C,T, and R that have different functions. The first crystal structure of the toxin was obtained in 1992 by x-ray crystallography. However, the structure was a dimer and generated by freezing the protein. The dimer is non-toxic but becomes a toxic monomer at neutral pH by dissociation. The of the molecule is made up of 24 helices, 31 beta sheets, and turns between each of them which adds up to be 535 residue protein. This makes for a mixture of that allow for the seondary structure. The purple portion of the molecule is the polar ares while the gray was the hydrophobic areas.
The two fragments are then separated into three domains C, T, and R which correspond to three major functions of the toxin.
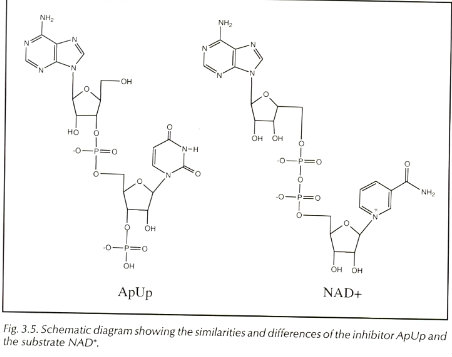
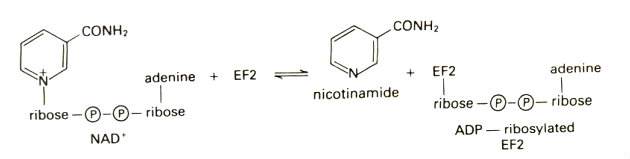
In the mechanism for Diphtheria toxin the R Domain binds to which are NAD+ which can sometimes be switched with ApUp. The molecule ApUp is bound to a cleft on the front side of the C domain. MechanismThe mechanism begins by the R domain recognizing the target cells by the molecules on the cells surface. A prominent β-hairpin loop of the R domain binds to the surface and allows for a docking station. The ligand that is typically bound on the cell surface is pro-HB-EFG. Binding leads to receptor mediated endocytosis. The T domain translocates the C domain into the cytosol of the cell. The organism Corynebacterium diphtheriae first secretes the toxin the loop between the C and T domain has to be nicked in order for the toxicity to be turned on. There is one surface protease named furin that is known to nick the area but it is unclear if any other protein helps the process. This process allows for the C domain to dissociate from the T domain after transported(translocated) into the cytosol. This is where the disulfide bonds become reduced. After this happens a drop in pH leads to the formation of the dimer to become an open monomer form. The reduction of the disulfide bonds is the rate determining step of the toxin entrying the cell. Once entried into the cytosol, the C domain does ADP-ribosylation of EF-2 at diphthamide, a posttranslationally modified histidine residue. This shuts down all protein synthesis and kills the cell. The Kcat/KM of the reaction is about 108 min-1 M-1. The figure below shows the reaction happening at the C domain. The C domain binds to the ligand NAD+ to begin the reaction. The C domian does have an inhibitor and that is ApUp which is near in the C domain.
Medical Implications & Possible ApplicationDiphtheria toxin is the causative agent of the disease Diphtheria. The lethal dose for humans and other susceptible eukarya is .1μg of toxin per kg of body weight[5] A vaccine has been made and is routinely used in first world countries as a basic immunization. The vaccine is made up of formaldehyde-treated Diphtheria toxin. However, Diphtheria toxin has been used is many other medical applications such as the drug Denileukin diftitox and cancer suppressors. Denileukin diftitox is a protein that combines Diphtheria toxin and Interleukin 2. In some Leukemias and Lymphomas cells express Interleukin receptors in which the drug can bind to and release the toxin within the cells.
References
Footnotes
|