Sandbox Reserved 492
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
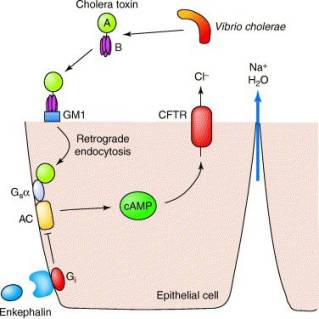
Cholix Toxin from Vibrio CholeraeThe crystal structure of the purified form of Cholix Toxin or CT was determined in 1995. [1] It is an oligomeric bacterial protein found to be made up of six individual subunits. V. cholerae toxin, along with other similar bacterial enterotoxins seem to share an evolutionary conserved composition comprising of about 13 alpha-helices and 10-12 Beta-sheets. The protein is then further divided into one single A-subunit and 5 individual B- subunits.The A-subunit makes up what is known as the enzymatic portion of the protein while the 5 copies of the B-subunit are responsible for the binding to the ligand receptor. The toxin binds highly specifically and tightly to a GM1 gangliosides on the surface of the host's cells. In this X-Ray Diffraction image we can see the site, which in this case has been complexed with an allosteric inhibitor (red and yellow space filling atoms). Recent studies have indicated several amino acid located proximally to the active site which are critical for enzymatic activity. Specifically, site directed mutagenesis indicated that when altered, the mutation results in a termination of the proteins toxicity, rendering it essentially harmless.
Toxin MechanismOnce secreted, the B subunit will bind to GM1 gangliosides on the surface. After binding takes place, the whole complex is engulfed by the cell and a portion known as the CTA1 chain is detached after reduction of a disulfide bridge. The new endosome is moved to the Golgi, where it is recognized by the endoplasmic reticulum, unfolded and delivered to the membrane, where the Endoplasmic Reticulum-oxidase - "Ero1" triggers the release of the excised A1 protein (through Oxidation) of protein disulfide isomerase complex. As A1 moves from the ER into the cytoplasm it refolds and avoids further reduction. [2]
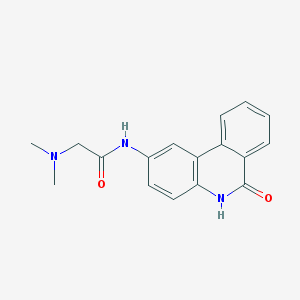
Studies & Potential BenefitsWhile CX intoxication from the bacterium is an incredibly serious and even life-threatening condition, tests conducted have shown that the toxin can be directly inhibited on the molecular level. The catalytic subunit of the CX protein is known to bind, with high affinity, to a molecule known as PJ34[5] - as well as with other structurally conserved, fused-hydrocarbon ring inhibitors. These types of interactions are studied through the use of computerized modeling, where factors such as Van der Waal radii, atomic radii, electron affinity etc.. are assessed producing viable molecular model.
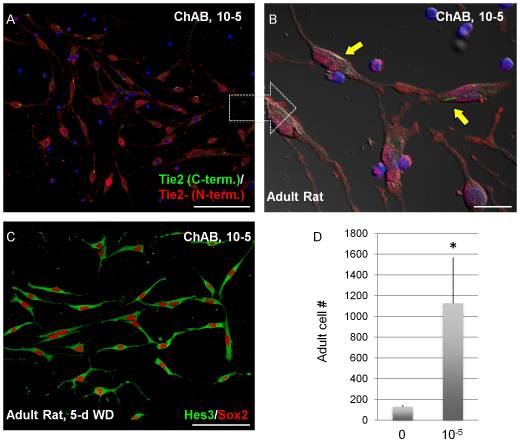
Essentially, the replaces what would be the target protein in the epithelial cell of a human thus prevents the Cholera Toxin from interacting with its normal ligand; this would in turn prevent the ill effects of intoxication...in theory. Cholix also serves several valuable purposes in Biological research. Dr. Pierre-Hervé Luppi and his laboratory recently discovered a new "tracing" method using cholera-toxin instead of the previously used classical molecules such as Horse Radish Peroxidase (HRP). In contrast to HRP, which is passively taken up by neurons, cholera-toxin (as we discussed previously) binds specifically to surface receptors of neurons and is can be actively taken up and transported by the axons. [6] This phenomenon helps enhance the sensitivity of cholera-toxin as a tracer, for studies dealing with neural function and the diseases known to affect it. As a result, aspirations for future research being done on Cholera Toxin today, coincide with a current "hot topic" within the science community and the entre World: Stem Cell Research. There have been some recent findings indicating that the protein may be capable of interacting - regulation on the genetic level - some key factors in Neural Stem Cell (NSC) regeneration and differentiation. Known as Tie2, a membrane receptor, and Hes3 a transcription factor, these two indicators have been shown to directly interact with the Cholix Toxin. Moreover, there are even some implications that the protein, when combined with specific medium, boosted Stem Cell culture growth.[7] Thus, we see that apart from its potential to cause human illness, CX also poses a solution to cancer and other related diseases.
References
|