This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 509
From Proteopedia
Protein Kinase R
Contents |
Abstract
One mechanism against viral infection preventing viral propagation by inhibiting cellular protein synthesis. The protein kinase R (PKR) is an several enzymes involved in cellular immunity against viral infection. PKR is composed of two domains: two double stranded RNA (dsRNA) binding domains and a kinase domain (KD). The crystal structure of the PKR KD is bound to its substrate, initiation factor eIF2α revealing that each KD is composed of N-terminal (N-lobe) and C-terminal lobes (C-lobe). The active site lies between these two lobes, which also contains an ATP. The two N-lobes of each PKR KD interact to form a dimer whereas the C-lobe is bound to eIF2α composed of an S1 domain and a helical domain. Upon viral infection, PKR senses the dsRNA inserted by the virus, and is dimerized and activated. The active PKR molecule then binds to eIF2α, causing a conformational change in eIF2α, bringing the helix insert containing Ser51 (phospho-acceptor residue) closer to the ATP. The γ phosphate of ATP is then transferred to the Ser51, and the phosphorylated eIF2α inhibits protein synthesis in infected cells. Such fundamental insights into the mechanisms of substrate recognition and phosphorylation by PKR will help design a small molecule that will activate PKR, leading to improved immunity against multiple viral infections. In addition, the mechanism of PKR function may be applied to cancer therapy due to its role in controlling cell differentiation.
Viral Defense against PKR Function
Viruses spread throughout the body by invading cells and hijacking the protein production machinery of the cell in order to replicate. However, there are several defense mechanisms used to counter viral infection. One is protein kinase R (PKR). In an uninfected cell, PKR exists as an inactive molecule. However, the presence of viral RNA triggers the PKR to form an active dimer. The active PKR then binds to an initiation factor eIF2α and transfers a phosphate from an ATP to the eIF2α, thereby inhibiting cellular and viral protein synthesis.
Viruses have evolved several mechanisms to subvert the PKR function (Figure 1). The VAI (Viral Associated I) RNA of adenovirus serves to prevent the dimerization and activation of PKR and inhibit the anti-viral response. Influenza virus p58IPK inhibits the activation of PKR. On the other hand, the smallpox virus K3L protein is a molecular mimic of eIF2α, acting as a competitive inhibitor and inhibits the phosphorylation reaction. Therefore fundamental understanding of this specific interaction between eIF2α and PKR and how viruses interfere with the reaction between the two is of paramount importance in developing anti-viral drugs.
Data Pieces
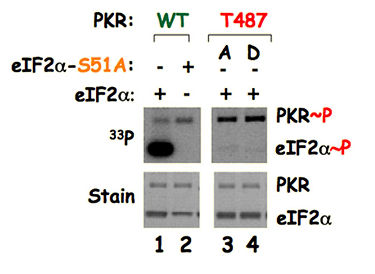
Lane 1: Wild type PKR, wild type eIF2α and 33Pγ-ATP were mixed and were resolved in a gel (lower panel stain). The gel was dried and autoradiographed (upper panel 33P). PKR transfers 33Pγ of ATP to itself (autophosphorylation indicated as PKR~P) as well as to the eIF2α indicated here as eIF2α~P). Lane 2: When wild type PKR, a mutated form of eIF2α-S51A (phosphorylation site is mutated to an alanine) and 33Pγ-ATP were mixed. PKR transfers 33Pγ of ATP to itself, but not to the eIF2α-S51A. These data confirm that the Ser51 residue is the phosphorylation site of eIF2α. Lanes 3 and 4: when PKR-T487A (PKR-T487D for the lane 4), wild type eIF2α and 33Pγ-ATP were mixed, PKR transfers 33Pγ of ATP to itself, but not to the eIF2α suggesting that the remote residue T487 is important for Ser51 phosphorylation. The residue T487 is located on the contact surface of PKR with eIF2α (Fig. 2), thus providing an experimental evidence of PKR-eIF2α structure and interaction at the remote region.
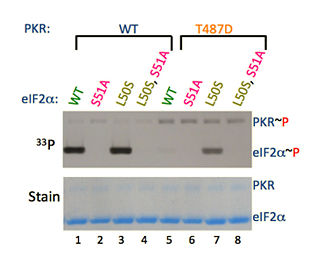
Figure on Left: Mutation of residue L50 that disrupts hydrophobic interaction causes a conformation change of Ser51 position. As a result PKR-T487D protein is able to transfer 33Pγ of ATP to the eIF2α-L50S, but not to the wild type eIF2α (as seen in the comparison of eIF2α~P lanes 5 and 7).
Figure on Right: A cluster of hydrophobic residues (L47, L50, I58 and I62) restricts the movement of Ser51 residue of eIF2α. These hydrophobic residues pull Ser51 in a hydrophobic pocket.
Conclusion
The mechanism of the PKR response to viral infection involves PKR activation (dimerization and autophosphorylation) and phosphorylation of eIF2α. To counteract PKR function, viruses have evolved specific mechanisms. Therefore understanding the structure and mechanism of PKR-eIF2α recognition will help design anti-viral drugs through preventing viruses from binding to PKR and through mimicking eIF2α’s function.
References
Dey, M., Velyvis, A., Li, J., Chiu, E., Chiovitti, D., Kay, L., Sicheri, F., Dever, T. E. Binding to PKR induces a conformational change in eIF2α to expose Ser-51 for phosphorylation. (2011) Proc. Natl. Acad. Sci., USA (in press).
Dey, M., Cao, C., Dar, A., Tamura, T., Ozato, K., Sicheri, F., and Dever, T. E. Mechanistic link between protein kinase PKR catalytic domain dimerization, autophosphorylation and eIF2α phosphorylation. (2005) Cell, 122, 901-913.
Dar AC, Dever TE, & Sicheri F (2005) Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell 122:887-900. Dhaliwal, S., Hoffman, D.W. J.Mol.Biol. (2003) 334: 187-195