This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 559
From Proteopedia
| This Sandbox is Reserved from 05/22/2012, through 07/22/2012 for use in the course "BIOL 414" taught by Greg Buhrman at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 551 through Sandbox Reserved 590. |
To get started:
More help: Help:Editing |
Hi Jacob, Here is your very own Proteopedia Page for pdb code: 2RH1. Your presentation is scheduled for: June 20.
Have Fun!
Greg Buhrman
| |||||||||
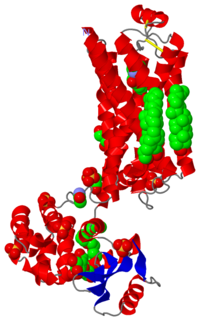
| 2rh1, resolution 2.40Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , , , , | ||||||||
| Gene: | ADRB2, ADRB2R, B2AR / E (Enterobacteria phage T4) | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
High resolution crystal structure of human B2-adrenergic G protein-coupled receptor.
Heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors constitute the largest family of eukaryotic signal transduction proteins that communicate across the membrane. We report the crystal structure of a human beta2-adrenergic receptor-T4 lysozyme fusion protein bound to the partial inverse agonist carazolol at 2.4 angstrom resolution. The structure provides a high-resolution view of a human G protein-coupled receptor bound to a diffusible ligand. Ligand-binding site accessibility is enabled by the second extracellular loop, which is held out of the binding cavity by a pair of closely spaced disulfide bridges and a short helical segment within the loop. Cholesterol, a necessary component for crystallization, mediates an intriguing parallel association of receptor molecules in the crystal lattice. Although the location of carazolol in the beta2-adrenergic receptor is very similar to that of retinal in rhodopsin, structural differences in the ligand-binding site and other regions highlight the challenges in using rhodopsin as a template model for this large receptor family.
High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor., Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC, Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
2rh1 is a 1 chain structure with sequence from Homo sapiens, enterobacteria phage t4. The April 2008 RCSB PDB Molecule of the Month feature on Adrenergic Receptors by David S. Goodsell is 10.2210/rcsb_pdb/mom_2008_4. Full crystallographic information is available from OCA.
See Also
- Beta-2 Adrenergic Receptor
- Group:SMART:A Physical Model of the β2-Adrenergic Receptor
- Group:SMART:Tangible Models of Cdc42 Interacting With Intersectin
- User:Frieda S. Reichsman
Reference
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007 Nov 23;318(5854):1258-65. Epub 2007 Oct 25. PMID:17962520
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007 Nov 23;318(5854):1266-73. Epub 2007 Oct 25. PMID:17962519
Categories: Adrenergic Receptors | Homo sapiens, enterobacteria phage t4 | RCSB PDB Molecule of the Month | ATCG3D, Accelerated Technologies Center for Gene to 3D Structure. | Cherezov, V. | Choi, H J. | Hanson, M A. | Kobilka, B K. | Kobilka, T S. | Kuhn, P. | Rasmussen, S G.F. | Rosenbaum, D M. | Stevens, R C. | Thian, F S. | Weis, W I. | 7tm | Accelerated technologies center for gene to 3d structure | Adrenergic | Atcg3d | Cholesterol | Fusion | Gpcr | Lipidic | Lipidic cubic phase | Membrane protein | Membrane protein / hydrolase complex | Mesophase | Protein structure initiative | Psi-2 | Structural genomic