Sandbox Reserved 591
From Proteopedia
| This Sandbox is Reserved from Feb 1, 2013, through May 10, 2013 for use in the course "Biochemistry" taught by Irma Santoro at the Reinhardt University. This reservation includes Sandbox Reserved 591 through Sandbox Reserved 599. |
To get started:
More help: Help:Editing |
Contents |
Background
Amyloid Precursor Protein (APP) is a major protein associated with Alzheimer’s disease (AD) and was first identified in 1987. It is located on chromosome 21 and has at least 18 exons. AD is characterized as the most common form of dementia that worsens over time. APP is produced in big amounts and is metabolized very quickly. Risk for developing AD increases with age. APP is produced in big amounts and is metabolized very quickly. APP was discovered in 1987 by several laboratories independently using partial protein sequence data. Alzheimer’s disease is caused by accumulation of β-amyloid peptide (Aβ) within the brain. Mutations in critical areas of APP cause familial susceptibility to AD. Over 25 APP mutations have been identified.
|
Structure
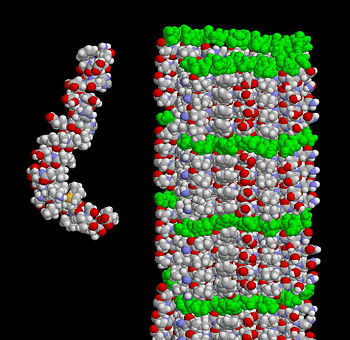
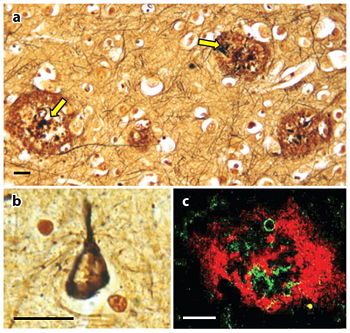
The extracellular region is separated into the E1 and E2 domains which are linked by an acidic domain. [1]. Over 70 percent of the amino acid residues of this region are involved with standard secondary structure elements. As shown in Figure 1, the amyloid beta peptide can be shown in two guises where the structure on the left shows how the peptide might look when it interacts with the membrane. A majority of it is folded into an alpha helix. The structure on the right displays the form in the amyloid fibril where thousands of peptides stack on top of each other to make an extensive beta sheet. Figure 2 shows different images of plaques on the neurites of a brain affected by AD. APP is a type I transmembrane that separates the large N-terminal extracellular domain from the short C-terminal cytoplasmic tail domain. The N-terminal, crystalized in 1999, contains nine beta-strands and only one alpha-helix. [2]. The domain has a surplus of cysteine and has three disulfide bridges and a hydrophobic core which together are well conserved throughout the APP family. The disulfide bridge between Cys98 and Cys105 stabilizes a β-hairpin loop which appears to be essential for neurite development and MAP kinase activation. This surface possibly represents one of the APP heparin-binding sites. Although, the β-hairpin loop is the most mobile area in this structure. [3]. Adjacent to this heparin structure is a hydrophobic surface patch which is critical in protein-protein interactions. The N-terminal head domain of APP has nothing in common with any other known protein. Since this structure can initiate neurite growth, some scientists think the N-terminal head domain alone is responsible for growth-factor like characteristics of APP. However, further exploration is needed to confirm this hypothesis. [4].
The cytoplasmic (intracellular) domain is released by a γ-secretase-dependent mechanism. Most known binding partners of APP interact with the intracellular domain. One of the most important is the YENPTY motif which is present in the AICD. This motif is important for Clathrin-mediated endocytosis and binding to many proteins like Fe65 and JIP. [5]. It seems that the release of AICD can contribute to intracellular variations in the cytoskeleton formation or kinase localization, and might even cause indirect effects on nuclear transcription. [6]Function
APP controls cholesterol turnovers needed for activity in the neurons. Some studies have even shown that it plays a role in memory deficiencies that many patients with AD experience. [7]. Most studies show overexpression of APP has effects on cell growth and health. The N-terminal heparin-binding domain of APP has been shown to stimulate neurite growth and promote synaptogenesis. Studies have also revealed that APP is able to regulate stem cells. APP plays a major role in the nervous system involved with synaptic plasticity. It also is key in maintaining neuronal calcium homeostasis which is important for transmitting synapses. [8]. One of the earliest indications of APP function came from analyzing the growth pattern of fibroblasts in which APP levels were lessened by expression of an antisense APP construct. These cells grew slowly, but the growth retardation could be restored by treatment with APPs. Along with the pentapeptide domain (RERMS), the infusion APPs into the brain resulted in a growth of synaptic size and improved memory capabilities in animals. An RHDS motif at the C-terminius of APP seems to promote cell adhesion. X-ray analysis revealed that the E2 domain of APP could form antiparallel dimers. Such a structure has the potential to function in trans-cellular adhesion. [9]. Experimental evidence has determined that APP controls the presynaptic expression and action of the high affinity choline transporter. In this circumstance, loss of APP leads to abnormal positioning of the choline transporter at neuromuscular junctions which could results in cholinergic impairment and AD pathogenesis. [10].
Clinical Relevance
Alzheimer's disease (AD) is the leading cause of dementia throughout the world which is characterized by the accumulation of β-Amyloid peptide (Aβ) within the brain. It affects one out of three individuals in elder people. These peptides are deposited as amyloid plaques in the brains of AD individuals. Researchers are trying to figure out ways to keep these plaques from forming because of majority of them believe that these plaques are responsible for the pathology of AD. Research suggests that the generation of Aβ from APP proteolysis is a crucial step in the development of AD. [11]. One APP knock out study performed on mice showed that the mice had deficits in forelimb grip strength and locomotor activity. This study also shows that APP plays a role in long-term memory in the adult brain and may be necessary during the development and maintenance of neuronal networks involved in this kind of memory. [12]. Aβ also leads to the neurodegenerative conditions seen in AD. [13]. It is suggested that disturbance of normal APP physiology can contribute to the development of AD. Even though no cure currently exists for AD, significant progress has been made in developing treatments for it. Future treatments are likely to include drugs that target steps in Aβ production. [14]References
'- ↑ Dahmes, Sven O et al. 2010. Structure and biochemical analysis of the heparin-induced E1 dimer of the amyloid precursor protein. PMC 107(12): 5381–5386
- ↑ Reinhard,Constanze. 2005. The amyloid-bold beta precursor protein: integrating structure with biological function. EMBO. 24: 3996 - 4006.
- ↑ Reinhard,Constanze. 2005. The amyloid-bold beta precursor protein: integrating structure with biological function. EMBO 24: 3996 - 4006.
- ↑ Reinhard,Constanze. 2005. The amyloid-bold beta precursor protein: integrating structure with biological function. EMBO. 24: 3996 - 4006.
- ↑ Zheng, Hui, Koo, Edward H. 2006. The amyloid precursor protein: beyond amyloid. PMC 1:1-12.
- ↑ O'Brien, Richard J. 2011. Amyloid Precursor Protein Processing and Alzheimer’s Disease. NIH. 34: 185–204.
- ↑ Octave JN, Pierrot N, Ferao Santos S, Nalivaeva NN, Turner AJ. From synaptic spines to nuclear signaling: nuclear and synaptic actions of the amyloid precursor protein. J Neurochem. 2013 Mar 16. doi: 10.1111/jnc.12239. PMID:23495999 doi:10.1111/jnc.12239
- ↑ Octave JN, Pierrot N, Ferao Santos S, Nalivaeva NN, Turner AJ. From synaptic spines to nuclear signaling: nuclear and synaptic actions of the amyloid precursor protein. J Neurochem. 2013 Mar 16. doi: 10.1111/jnc.12239. PMID:23495999 doi:10.1111/jnc.12239
- ↑ Zheng, Hu, Koo, Edward H. 2006. The amyloid precursor protein: beyond amyloid. PMC 1:1-12.
- ↑ Kudoh Y, Shiiki M, Sasa Y, Hotta D, Nozawa A, Iimura O. Slow continuous hemodialysis--new therapy for acute renal failure in critically ill patients--Part 2. Animal experiments and clinical implication. Jpn Circ J. 1988 Oct;52(10):1183-90. PMID:3210295
- ↑ Zheng, Hui, Koo, Edward H. 2006. The amyloid precursor protein: beyond amyloid. PMC 1:1-12.
- ↑ Senechal Y, Kelly PH, Dev KK. Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav Brain Res. 2008 Jan 10;186(1):126-32. Epub 2007 Aug 9. PMID:17884188 doi:10.1016/j.bbr.2007.08.003
- ↑ Zhi-dong Zhou. 2011. The roles of amyloid precursor protein (APP) in neurogenesis, implications to pathogenesis and therapy of Alzheimer disease (AD). PMC 5(4): 280–92.
- ↑ O'Brien, Richard J. 2011. Amyloid Precursor Protein Processing and Alzheimer’s Disease. NIH. 34: 185–204.