Sandbox Reserved 640
From Proteopedia
Contents |
Leghemoglobin
|
Introduction
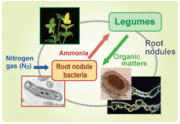
Leghemoglobin is a protein that is required by Legume plants in order to fix nitrogen by participating in the Nitrogen Fixation pathway. It is found in legumes such as cowpea, soybeans, alfalfa, and other different types of beans. Leghemoglobin functions by keeping the oxygen at a specific low concentration to the bacteroids to allow for respiration, but at the same time, this low concentration keeps the oxygen out of the nitrogen cycle so it doesn’t inhibit the nitrogenase activity [1]
Leghemoglobin is a 16 kD globin protein containing eight secondary alpha helices, constituted by a heme group(protoporphyrin XI) and a single polypeptide. In its ferrous (reduced state), Leghemoglobin binds oxygen for transportation. [2]. It is hypothesized that Leghemoglobin can be formed by one of the two ways:
- The Rhizobium bacteroid [3] is thought to synthesize the heme group in the root nodules of the legume, where it then gives the plant the heme to complete the synthesizing of the Lb [1]
- The plant itself has also been thought to possibly produce the heme itself, in the mytochondria of the plant cells. It then combines with the peptide sequence to complete the whole protein [1]
Function
In order to fix nitrogen, legume nodules require Leghemoglobin. In 1958, Bergersen and Appleby showed that only the ferrous form of Leghemoglobin binds to oxygen in the cytoplasm of the infected plant cells. [1]. Therefore, the function of Leghemoglobin is to carry the oxygen for the bacterial respiration, as well as for nitrogen fixation.
The enzyme needed for nitrogen fixation is nitrogenase. However, this enzyme is inactivated by oxygen, but at the same time, oxygen is needed for the bacteria to reduce nitrogen to ammonia. This ambiguity is explained by the function of Leghemoglobin, which transports oxygen at a low but stable concentration allowing for the symbiotic operation of nitrogenase activity and bacterial respiration.
Structure
|
The 16 kDa polypeptide leghemoglobin consists of two main subunits, the main globin structure and the iron-encompassing heme (protoporphyrin XI) group. These two subunits form a molecule structurally similar to Myoglobin [1]. The globin fold portion of the molecule is the standard globin secondary structure, a series of [2]. Depending on the species and specific type of Leghemoglobin, the primary amino acid sequence can differ some, but overall it is well conserved.
The prosthetic group consists of four 5-membered pyrrole rings, forming a cyclic ring around a central iron (Fe) atom. This atom is contained within four equatorial nitrogens, in addition to another nitrogen from a close histidine residue and an opposite dioxygen [4]. The ligand contact residues along the heme group are Phe30, , and Val67. Depending on the specific type of Leghemoglobin, some of these specific residues are disordered and others interconvert between two conformations [5].
In opposition to the differences in polypeptides among globin proteins (Hemoglobin and Myoglobin), the heme group has been found to remain largely the same[1]. The main difference is a considerably larger heme group in Leghemoglobin than its other oxygen-transferring counterparts. The way the heme group attaches to the polypeptide is also different. The steric crowding around the ligand binding site (beside the heme) is reduced, plus there is an altered packing at the proximal side of the heme and conformational differences along the distal side. As a result, the oxygen affinity is larger than that of Myoglobin and Hemoglobin [6]. This is reflected by the Km of 0.01 microM (the concentration for half of the Leghemoglobin to be saturated with dioxygen), about ten times the Michaelis constant for Hemoglobin [5]).
The methods by which various Leghemoglobins were purified, and then analyzed, are as follows [1]:
- Ammonium Sulfate Precipitation
- Concentration by Ultrafiltration
- Electrophoresis
- Anion-exchange Column Chromatography
- Isoelectrofocusing
- Nuclear Magnetic Resonance (NMR)
- X-ray crystallography
Mechanism
Because Leghemoglobin can autooxidize, there are several mechanisms to maintain the protein in its active (reduced) form. One of them involves an enzyme called, Leghemogobin Reductase[1]. This protein is a flavoprotein that catalyzes the reduction of Lb3+ (ferric form) to Lb2+ (ferrous form) using NADH. The reaction is as follows:
Ferric Leghemoglobin can also be reduced to the ferrous form by free flavins in the presence of NADH or NADPH. Lastly, Leghemoglobin can be reduced to its ferrous form directly by physiological reductants. These reductants are various electron donors commonly found in plant cells, which reduce the Leghemoglobin nonenzymatically.[3].
Implications and Applications
Microorganisms that fix nitrogen are known as diazotrophs. The main problem occurring for these organisms is the oxygen that interferes with nitrogenase, the enzyme that converts the nitrogen to ammonia. The problem lies in the fact that some organisms need oxygen to live, but need it not interfere with nitrogenase. Others fare better by living in low oxygen environments, so no help is necessary [7]
The diazotrophs are split into two categories, one being free-living and the other symbiotic. The free-living diazotrophs don’t need Leghemoglobin for nitrogen fixation as they have their own way of staying away from oxygen. These evolutions could vary, including living in low-oxygen areas or respiring oxygen quickly enough to keep levels low. [7] [8]
Organisms that use Leghemoglobin form root nodules that are important symbiotically with different types of bacteria. The legume family Fabecea consists of the species using Leghemoglobin, with some minor exceptions. Examples of organisms in this family are clovers, soybeans, alfalfa, lupines, and peanuts [7] [8].
Other nitrogen fixing plants don’t need oxygen, or need very trace amounts, for nitrogen fixation. Some need heterocysts, which are specific nitrogen-fixing cells, and have no need for Leghemoglobin because of its own anti-oxygen evolved state. Examples of what could replace Leghemoglobins are proteins that are specific to scavenging oxygen out of the cells, as well as having multiple cell walls, one of which is a glycolipid, that helps to keep out oxygen [7].
Nitrogen Fixation is essential for Crop Rotation, as some plants need nitrogen and others assist in nitrogen fixation that can provide it. The legumes play an important role in this as they can be rotated with vegetable crops that need a renewable nitrogen source. Maize is one of these crops that is perfect to rotate with soybeans to maintain healthy soil. Otherwise, the corn would diminish the nitrogen and the land would be unable to grow more. The soybeans are grown on the same soil afterwards to replenish the nitrogen. The corn is then rotated back in to have a fruitful harvest.
Notes
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Becana, M., Moran, J.F., Iturbe-Ormaetxe, I., Gogorcena, Y. and Escuredo, P.R. 1995. Structure and function of leghemoglobins. An. Estac. Exp. Aula Dei (Zaragoza) 21(3): 203-208.
- ↑ 2.0 2.1 Vinogradov, Serge, David Hoogewijs, and Xavier Bailey. "A Model of Globin Evolution." National Center for Biotechnology Information. U.S. National Library of Medicine, 4 May 2007. Web. 06 Nov. 2012. <http://www.ncbi.nlm.nih.gov/pubmed/17540514>.
- ↑ 3.0 3.1 Appleby, C. A. "Leghemoglobin and Rhizobium Respiration." Annual Review of Plant Physiology 35.1 (1984): 443-78. Print.
- ↑ TN Safonova, AV Teplyakov, GV Obmolova, AN Popov, IP Kuranova, EG Harutyunyan. 1991.Crystal structure of ferric complexes of the yellow lupin leghemoglobin with isoquinoline at 1.8 angstroms resolution (Russian). Bioorg. Khim. 17:1605.
- ↑ 5.0 5.1 Narula, Surinder S., Claudio Dalvit, Cyril A. Appleby, and Peter E. Wright. "NMR Studies of the Conformations of Leghemoglobins from Soybean and Lupin." European Journal of Biochemistry 178.2 (1988): 419-35. Print.
- ↑ Ellis, P. J., C. A. Appleby, J. M. Guss, W. N. Hunter, D. L. Ollis, and H. C. Freeman. "Structure of Ferric Soybean LeghemoglobinNicotinate at 2.3 Å Resolution." Acta Crystallographica Section D Biological Crystallography 53.3 (1997): 302-10. Print.
- ↑ 7.0 7.1 7.2 7.3 Postgate, J (1998). Nitrogen Fixation, 3rd Edition. Cambridge University Press, Cambridge UK.
- ↑ 8.0 8.1 Smil, V (2000). Cycles of Life. Scientific American Library.