Sandbox Reserved 641
From Proteopedia
Contents |
Glutamate Dehydrogenase
|
Glutamate dehydrogenase (GDH)is an enzyme found in the mitochondria of most organisms. GDH is used to remove the ketone group and replace it with an α-amine group on the α-carbon, which forms glutamate. Glutamate is one of the 20 essential amino acids. This is done in reverse to supply α-ketoglutarate to the tricarboxylic acid (TCA) cycle. GDH is an oxidoreductase, which is an enzyme that transfers electrons from one molecule (reductant/electron donor) to another molecule (oxidant/electron acceptor).
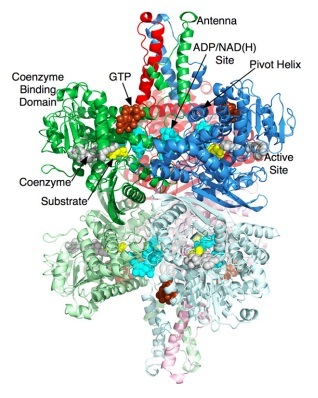
Glutamate dehydrogenase is a hexamer that contains two domains that have three subunits. GHD contains approximately 18 alpha helices and thirteen beta sheets. There is a large cleft that separates the two domains and allows for a substrate to enter and bind. The protein then closes around the substrate. For mammals only there is a structure that extends outward of the protein called the "antennae." (8)
Today, one of the primary research uses for glutamate dehydrogenase is to determine how well the human liver is functioning. If the level of GDH is too high that could indicate necrosis of the liver. (7)
Structure
Glutamate Dehydrogenase is a hexamer that is comprised of two trimer subunits. These two subunits are stacked on top of each other and composed of three domains. The top of each domain contains a "NAD-binding domain" that has the conserved nucleotide-binding motif. A larger helix-loop-helix structure rises above this and is referred to as an "antenna." This antenna contains approximately 50 amino acids and is thought to play a major role in regulation of the enzyme. This antennae structure is only found in animals. (1) The bottom domain contacts a domain in the other trimer, holding the two trimers together. The total size of each of the subunits is approximately 56.1 kD and 506 amino acids long. (5)
When a substrate binds to the enzyme it binds to the deep recess of the cleft between the NAD binding domain and the lower domain. Along the outside surface of the cleft a coenzyme (NAD+) binds to the C-terminal end causing the binding domain to rotate by about 18 degrees and close down on the substrate and coenzyme. (2) The active sites for GDH are located at residues around 182-187. Residues at the locations of 200-206, 375-370, and 421-423 are involved in closing the cleft between the domains. As the cleft is closing the antenna pushes against the pivot helix of the adjacent subunit. The pivot helix rotates counter clockwise around both the helical axis and the trimer 3-fold axis. The hexamer then compresses the inner core showing that catalysis involves the entire hexamer. (8)
The figure on the right shows the two domains of GDH. The orange represent the glutamate binding sites and the blue sites represent the domain involved in assembly of the hexamer.
Methods for Purification:
Ammonium Sulfate Precipitation,
Ion Exchange Chromatography,
Affinity Chromatography on a column of allosteric inhibitor bound the Sepharose (6)
Methods for Solving the Structure: single-crystal X-ray-diffraction
Mechanism
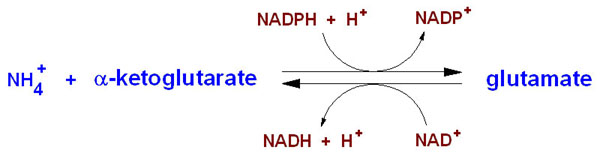
NH4+ + α-ketoglutarate + NADPH + 2 H+ → glutamate + NADP+ + H2O
Glutamate dehydrogenase is important in nitrogen and glutamate metabolism and energy homeostasis. In the reaction above the forward reaction is essential in converting free ammonia and α-ketoglutarate to glutamate, an amino acid that is used for protein synthesis. The reverse reaction is key reaction that links amino acid metabolism with the Tricarboxylic Acid cycle (TCA cycle). Both reactions utilize nicotinamide nucleotide cofactors: NAD+ when nitrogen is released and NADPH when nitrogen is used. Glutamate dehydrogenase is regulated by cell energy charge. This requires Adenosine triphosphate (ATP) and Guanosine triphosphate (GTP) are positive allosteric effectors for the forward reaction and Adenosine diphosphate (ADP) and Guanosine diphosphate are positive allosteric effectors for the reverse reaction. When the level of ATP is high, conversion of glutamate to α-ketoglurate and other TCA cycle intermediates is limited; when the cellular energy charge is low, glutamate is converted to ammonia and oxidizable TCA cycle intermediates. Glutamate is an important amino acid since it gives an amine group for many transamination reactions, thus, glutamate dehydrogenase is essential in producing this amino acid. (9)
Applications
Glutamate dehydrogenase is a mitochondrial enzyme present in the liver and can be used to determine how well the liver is functioning. Blood serum levels are measured and if levels are high it could be indicative of hepatocellular necrosis. Liver diseases in which necrosis of heptocytes are involved, such as toxic liver damage hypoxic liver disease, are characterized by high serum GDH levels. GDH is important for distinguishing between acute viral hepatitis and acute toxic liver necrosis or acute hypoxic liver disease, particularly in the case of liver damage with very high aminotransferases. GDH is measure by conducting the following reaction. (7)
- GDH
a-oxoglutarate + NADH + NH4+ ---------------> glutamate + NAD+ +H2O
As NADH is oxidized, the decrease in the absorbance per minute is measured spectrophotometrically at 340nm and is proportional to the GLDH activity.
An example of this can be seen here: [1]
Notes
(1) Smith, Thomas J., and Charles A. Stanley. "Untangling the Glutamate Dehydrogenase Allosteric Nightmare." Trends in Biochemical Science 33.11 (2008): 557-564. Print.
(2) Franco, Ann. "Reaction Mechanism of L-Glutamate Dehydrogenase." European Journal of Biochemistry 45(1974): 407-424. Print.
(3) Baker, Patrick J, et. all. "Subunit Assembly and Active Site Location in the Structure of Glutamate Dehydrogenase." Proteins: Structure, Function and Genetrics 12(1992): 75-86. Print.
(4) Smith, Thomas J., and Peter E. Peterson. "The Structure of Bovine Glutamate Dehydrogenase Provides Insights into the Mechanism of Allostery." Structure 7.7 (1999): 769-782. Print.
(5) Smith, Emil L, et. all. "Bovine Liver Glutamate Dehydrogenase: Tentative Amino Acid Sequence; Identification of a Reactive Lysine; Nitration of a Specific Tyrosine and Loss of Allosteric Inhibition by Guanosine Triphosphate." Proceedings of the National Academy of Sciences 67.2 (1970): 724-730. Print.
(6) Godinot, Catherine, et. all. "A Rapid and Efficient New Method of Purification of Glutamate Dehydrogenase by Affinity Chromatography on GTP-Sepharose." Analytical Biochemistry 61.1 (1974): 264-270. Print.
(7) Randox. "Glutamate Dehydrogenase (GLDH) for the Differential Diagnosis of Liver Disease." (2007): N. pag. Print.
(8) Minter, Mellisa. "Glutamate Dehydrogenase." Oxidoreductases And The Reactions They Catalyze. University Of Wisconsin-Eau Claire, 2005. Web. 08 Nov. 2012. <http://www.chem.uwec.edu/Webpapers2005/mintermm/index.html>.
(9) King, Michael. "Nitrogen Metabolism." The Medical Biochemistry Page. Themedicalbiochemistrypage.org, 2012. Web. 08 Nov. 2012. <http://themedicalbiochemistrypage.org/nitrogen-metabolism.php>.