Sandbox Reserved 648
From Proteopedia
ǣ
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||||||||||||||||||||||||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
N-Acetylglutamate synthase
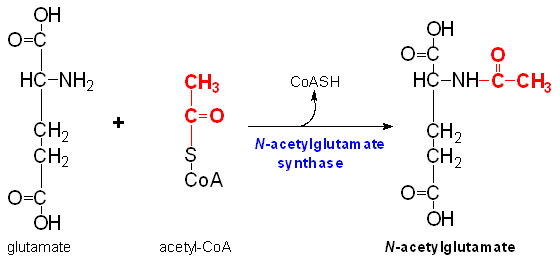
IntroductionN-Acetylglutamate synthase (NAGS) is a mitochondrial enzyme involved in the Urea Cycle. The urea cycle is responsible for producing urea from toxic ammonium. NAGS is most directly used in the conversion of glutamate (glutamic acid) and Coenzyme A into N-Acetylglutamate (NAG). N-Acetylglutamate synthase was first discovered as a mammalian liver enzyme but has very low rate of conservation across phyla. The function of NAGS changed throughout evolution from catalyzing the first reaction of arginine biosynthesis in microorganisms to production of an essential activator for Carbamoyl phosphate synthetase I in ureogenic organisms [1]. In mammals, N-Acetylglutamate synthase produces the enzyme cofactor N-Acetylglutamate which modulates Carbamoyl phosphate synthetase I activity. Carbamoyl phosphate synthetase I (CPSI) is the first rate limiting enzyme in the Urea cycle. L-arginine, ammonium, and nutrients derived through the diet can regulate NAG production through NAGS. L-argninie up regulates the activity of NAGS in mammals. Between vertebral species, 197 amino acids are identical in the current NAGS alignment. In humans, NAGS is synthesized by 534 amino acids that make up a pre-protein but between humans and mice this sequence is only 63% identical. Unlike mammalian NAGS, in bacteria and fungi NAGS is inhibited by arginine. This is because in these microbes NAGS is responsible for catalyzing the production of the the first committed intermediate of arginine biosynthesis. This difference is likely a result of an evolutionary change as tetrapods from the sea moved onto land. StructureIn 2009, scientists were able to crystallize and solve the three-dimensional NAGS structure from Neisseria gonorrhoeae (ngNAGS), which is closely related to E. coli and other bacterial NAGS proteins (50–60% sequence similarity), but has low sequence similarity (20–30%) to mammalian NAGS [2]. Mammalian NAGS proteins have a variable domain that is 35–40 amino acids long, rich in charged amino acids and prolines, and likely devoid of a [3]. While the NAGS enzyme is not over all phyla, in general the alpha helices, beta sheets and turns allow for and hydrophilic areas which allow for the folding of the secondary structure. When viewing the 3D model to the right, is is apparent that NAGS folds into two distinct domains. The kinase domain binds arginine while the acetyltransferase domain contains the catalytic site [4].
Mechanism of action
Medical Implications or Possible ApplicationNAGS deficiencies: Because of the nature of N-Acetylglutamate synthase, symptoms of NAGS deficiency present similarly to a CPSI deficiency. In both deficiencies patients will have elevated plasma ammonia and glutamine, reduced or absent citrulline, and normal urinary orotate [6]. Overall this commonly presents as hyperammonemia in humans. To tell the difference between the two, an enzyme assay with CPSI and NAG is done. Patients with NAGS deficiency will show normal CPS activity. It is possible to treat NAGS deficiencies with a structural analog to NAG. For many patients, N-carbamylglutamate can be used to restore proper CPSI activity. References
Footnotes
|
|||||||||||||||||||||||||||||||||||||||