Sandbox Reserved 652
From Proteopedia
| This Sandbox is Reserved from 30/08/2012, through 01/02/2013 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 636 through Sandbox Reserved 685. | |||||||||||||||||||||||||||||||||||||
To get started:
More help: Help:Editing For more help, look at this link: http://proteopedia.org/w/Help:Getting_Started_in_Proteopedia
Alanine Aminotransferase
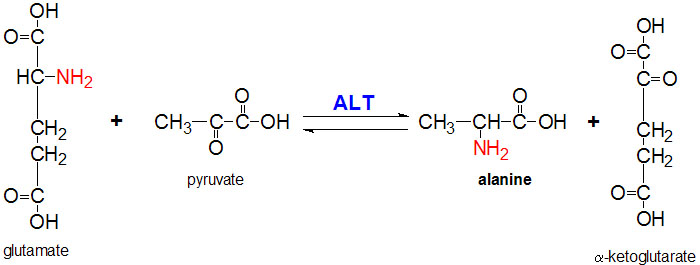
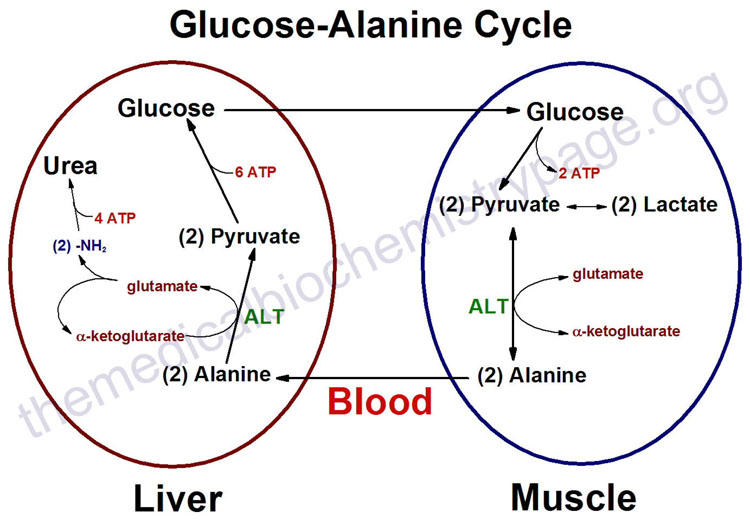
IntroductionAlanine aminotransferase (ALT), also known as L-alanine:α-ketoglutarate aminotransferase (EC 2.6.1.2); and also formerly known as alanine transaminase, is an intracellular cytoplasmic enzyme, which is found in greatest quantities in the liver and kidneys. ALT's main function is to catalyze the transfer of amino groups from alanine to form pyruvate from α-ketoglutarate, which is a key intermediate of gluconeogenesis. Levels of ALT are often quantified in humans to measure liver damage from inflammation or necrosis. High levels of ALT could be a response to high alcohol intake, obesity, diabetes, and other liver conditions. Quantification of ALT is limited because it is only able to identify liver damage but not the cause of the liver damage. StructureALT is a single polypeptide chain that is 498 residues long. ALT's secondary structure is includes 21 helices that make up 43% of the proteins overall structure as well as 21 beta sheet strands that make up another 16% of ALT's secondary structure. This composition places ALT in the alpha and beta (a/b) class of proteins. ALT's main domain is comprised of three layers in an a/b/a fashion. The beta layer is made of 7 mixed beta-sheet strands in a configuration where strand 7 is antiparallel to the rest. Two ligands are required for the proper structure and function of ALT. The first being the metabolically active form of vitamin B6, pyridoxal 5'-phosphate (PLP). PLP plays a vital role as a cofactor in many enzymatic processes that occur in the human body including amino acid metabolism, gluconeogenesis, lipid metabolism, and neurotransmitter synthesis. The other ligand utilized by ALT is the phosphate ion (PO4-). Mechanism of ActionImplications and ApplicationsQuantitation of ALT involves the coupled reaction involving lactate dehydrogenase, which oxidizes pyruvate to lactate. During this reaction pyruvate is reduced by NADH. The reduction in absorbance at 340 nm (NADH->NAD+) is proportional to the relative concentration of ALT in the coupled reaction depicted in the reaction equation above. The ALT levels quantified give a general status of the liver's condition. High levels of ALT are usually involved in severe liver damage, while significantly low levels of ALT can occur in cholestasis. When used in the context of liver disease, ALT can be used in an Aspartate Aminotransferase (AST)/ALT ratio. A ratio greater than two suggests alcohol as a cause of the liver disease, while a ratio less than or equal to one suggests a non-alcoholic cause. Levels of ALT in different parts of the body, such as the kidney, can also indicate damage to the hepatic system. References[1] http://www.acb.org.uk/docs/NHLM/ALT.pdf [2] http://www.rcsb.org/pdb/explore/explore.do?structureId=3IHJ [3] http://www.uniprot.org/uniprot/P24298 [4] http://scop.berkeley.edu/sunid=115355 [6] |
|||||||||||||||||||||||||||||||||||||


 ]
]
