Sandbox Reserved 690
From Proteopedia
| This Sandbox is Reserved from 30/01/2013, through 30/12/2013 for use in the course "Biochemistry II" taught by Hannah Tims at the Messiah College. This reservation includes Sandbox Reserved 686 through Sandbox Reserved 700. |
To get started:
More help: Help:Editing |
| Please do NOT make changes to this Sandbox. Sandboxes 30-60 are reserved for use by Biochemistry 410 & 412 at Messiah College taught by Dr. Hannah Tims during Fall 2012 and Spring 2013. |
|
Contents |
TRPV1
TRPV1[1] is also known as the capsaicin recpetor and VR1(vanilloid receptor 1). TRPV1 stands for transient receptor potential cation channel subfamily V member 1. TRPV1 is a protein responsible for detecting and regulating body temperature. It also is a nociceptor, meaning it also provides the sensations of heat and pain to the body. TRPV1 is found primarily in the dorsal root ganglia neurons of the body.
Please note that images are from an analog of TRPV1, which scientists then modify on computers to turn into TRPV1. As I do not have the software nor time to do so, I will be using the analog to discuss TRPV1 referencing it's active sites and such in the box while explaining the active sites of TRPV1. To reiterate, this is not TRPV1. We are just pretending it is.
Structure
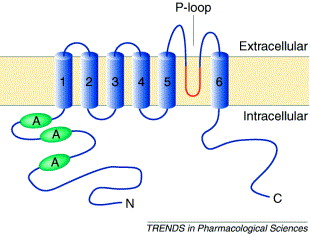
TRPV1 consists of 6 transmembrane domains. In the image of actual TRPV1 given, they are characterized by different colors.The "TRPV1" image also shows 3 distinct protein groups, A is shown in red, B is shown in green, and C is shown in blue. One can see, the of TRPV1 contains both beta sheets and alpha helices, with the alpha helices being the ones that cross the membrane.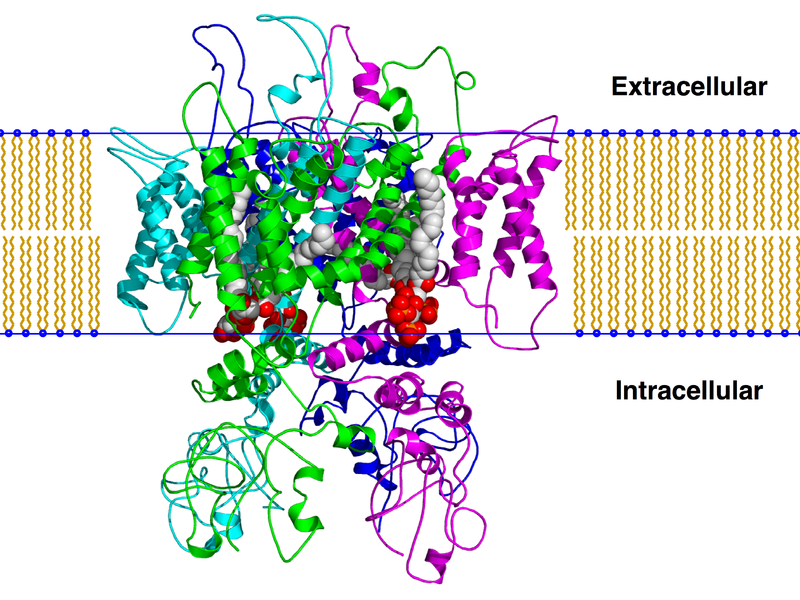
As TRPV1 is a transmembrane protein, it consists on both and hydrophillic residues. The hydrophobic regions are shown in blue, and hydrophillic in red. Between the 5 and 6 domains of TRPV1 there consists a hydrophobic channel in which the cations flow through(Shown in the image below).
Hydrogen bonds did not work. so i didn't make an awkward non-working link.
Interactions and Active Sites
TRPV1 responds to a variety of of agonists and antagonists. Agonists include capsaicin[2], protons and heat, gonists include CaM, ATP, and CaMKIII. The sites of , shown in lime green, occur on the C subunit (shown at the top) and the A subunit (bottom). The interractions of protons and capsaicin occur on the C subunit, and many of the regulation sites and heat become active on the A subunit. As this is not the real TRPV1 receptor, I am unable to show specific binding sites, however capsaicin binds to Arg 114, Tyr 511, Ser 512, Thr 550 and Glu 761 of TRPV1, all of which occur on the intracellular portion of the protein on the 3 and 5 domains. Protons interact with to Glu 600 and Glu 648, on the outer portion of the protein. Heat promotes its effects through interaction with the distal half of the C-terminus. Concerning regulation, CaM binds to Lys 155, ATP to Lys 155, Lys 160 and Leu 163, and CaMKII to Ser 502 and Thr 704.
Mechanisms
The mechanism for many of the agonists occurs through various external stimuli. In the case of capsaicin, the chemical comes from the foods we eat. This chemical is then bound to the aforementioned sitesInteractions and Active Sites which signals for the channel to become open. This opening allows the influx of cations to flood into the cell, thereby releasing an action potential which signals the neuron to sense heat and pain. Similar mechanisms exist for both protons and heat as well. Recent studies[3] show that these three agonists act in cohesion, with protons and capsaicin lowering the threshold for the action potential for heat to generate a reaction.
Antagonists such as CaM use feedback mechanisms to regulate the effect of agonists on TRPV1. Using the polarization of TRPV1 as a clue, agonists bind to their active sites on the N-Terminus on TRPV1, thereby closing the channel between domains 5 and 6. This causes TRPV1 to reduce it's signal of heat and pain, also allowing the buildup of cations on the outside of the cell to exist once again.. This is feedback is also why there is an assumed numbing effect when one eats spicy foods, as these antagonists cause desensitization of pain.
Images below show generic mechanism of agonist and antagonist interaction, as due to the nature of my protein modeling I am unable to show such interactions through animation.
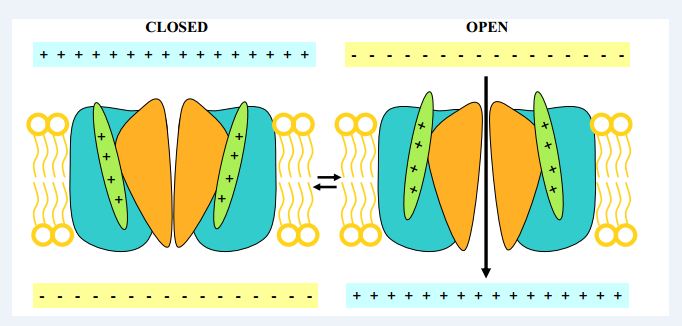 Image showing the opening and closing of channel. This is determined by agonist and antagonist interaction.
Image showing the opening and closing of channel. This is determined by agonist and antagonist interaction.
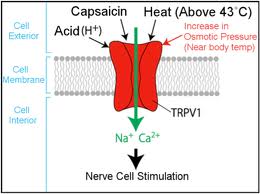 This shows a generic mechanism of agonist binding to TRPV1. Note that capsaicin and heat actually interact on the inside of the protein, not outside as shown.
This shows a generic mechanism of agonist binding to TRPV1. Note that capsaicin and heat actually interact on the inside of the protein, not outside as shown.
Inhibitors
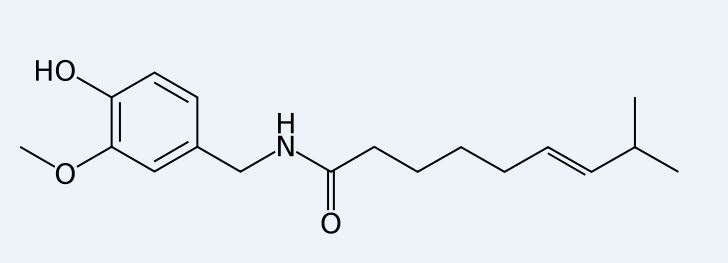
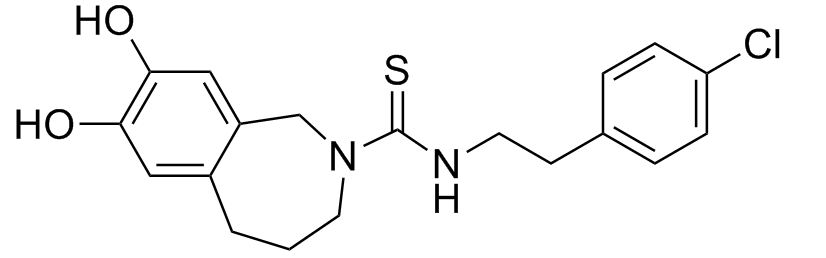
Capsazepine[4] and ruthenium red[5] have been shown to be inhibitors of TRPV1[6]. Capsazepine acts as a competitive ihibitor to capsaicin, thereby binding on its receptor and active sites. Ruthenium red is a calcium channel blocker. Therefore by inhibiting calcium from flowing across the channel the signal is prevented from activating. These inhibitors have an effect on heat as well, thereby showing the interaction of agonists with each other.
The images below show the structures of capsaicin and capsazepine, thereby showing their similarity.
References
All information and images have been taken from my paper, Google Images, or Wikipedia, and have been cited appropriately in my paper or presentation. The image of channels has been taken from http://www.benthamscience.com/open/topainj/articles/V003/SI0066TOPAINJ/68TOPAINJ.pdf.