Sandbox Reserved 760
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
|
Contents |
INTRODUCTION
What comes to the mind when one thinks about nitrogenase is the nitrogen cycle in which nitrogen is converted from its naturally occurring form to ammonia. Biological nitrogen fixation, the reduction of atmospheric N2 to NH3 by nitrogen fixing organism, is a key step in the nitrogen fixation pathway.[1] Nitrogenase is an enzyme (protein) that fixes nitrogen (N2) from the atmosphere into ammonia (NH3). This enzyme is only found in prokaryotic organisms such as certain bacteria and blue-green algae in which some are free living (Cyanobacteria) or symbiotic (Rhizobia) in nature.
Nitrogenase catalyzes the following reaction: N2 + 8H+ + 16MgATP + 8e− → 2 NH3 + H2 + 16MgADP + 16Pi.
Eight electrons are supplied to the reaction via the reduction of nitrogenase coupled by the presence of twice the amount of ATP and magnesium ions. The substrate (N2) is then reduced in the active site of the enzyme along with 8hydrogen ions to form 2 ammonia molecules and a by-product of hydrogen. The hydrolyzed ATP gives off ADP and inorganic phosphate. This process is catalysed by nitrogenase, the most common form of which is the molybdenum nitrogenase. The molybdenum nitrogenase is made up of dinitrogenase also known as Molybdenum-iron protein (MoFe protein) or NifDK, a double α and double β tetramer encoded by the nifD and nifK genes, respectively.[2] Molybdenum nitrogenase has another protein, dinitrogenase reductase also known as iron protein (Fe protein) or NifH, a γ2 homodimer encoded by the nifH gene. The NifH protein is responsible for ATP-hydrolysis-driven electron transport to the NifDK protein, which contains the site for the reduction of dinitrogen to ammonium. The reduction of N2 to two molecules of NH3 is an energy consuming process that requires 16ATPs, 2ATPs per electron used in the reaction.[3]
General information
Organism: Prokaryotes like bacteria and cyaanobacteria
Classification: Oxidoreductase
Structure: Fe protein: Dimer,FeMo Protein: 2alpha/beta tetramer
Length: Fe protein: 289 residues,FeMo protein: alpha: 492 residues and beta: 523 residues
Molecular Weight: Fe protein:63.662 KDa, FeMo protein: 235.154KDa
STRUCTURE AND FUNCTION
The first method for crystallization of nitrogenase was put forward by Burns R.C., Holsten R.D. and Hardy R.W., then was modified by Shah V.K. and Brill W.J. and was simplified by the 7th Laboratory, Institute of Botany, The Chinese Academy of Sciences. But the crystals gotten from these laboratories were small and needle-shaped therefore wasn’t suitable for X-ray diffraction analysis.[4] Weininger M.S. and Mortenson L. E. attempted to do X-ray diffraction analysis of MoFe protein crystals grown by using microdialysis method but they did not succeed in analyzing the crystallographic structure of MoFe protein. Later Kim and Rees succeeded in growing the big crystals of Azotobacter vinelandii nitrogenase by the microcapillary batch method and in analyzing of these crystals by X-ray diffraction. Since then, the liquid/liquid diffusion method has been usually used for crystal growth of nitrogenase proteins.[5]
Fe protein
The Fe-protein is an approximately 60 kilo dalton dimer of identical subunits with a single cluster of Fe4S4 in between the two subunits.[6]
The three-dimensional crystal structure of Fe-protein symmetrically coordinated from each subunit. Significantly, the subunits have the α-helical and β-sheet type of shape commonly associated with a major class of nucleotide-binding proteins. The two subunits are related by a molecular two-fold rotation axis that passes through the iron-sulfur cluster, which is located at the center of the dimer, right at the interface of the subunits.The iron-sulfur cluster not only serves as a crosslink between both subunits but it helps stabilize the dimer structure along with some salt and hydrophobic interactions. The Fe protein has two main functions; one: binding MgATP, MgADP, and nucleotides.[7]
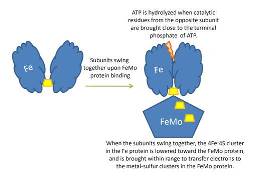
Two: the second functional site in the Fe protein is the Fe4S4 cluster, which undergoes a one electron redox cycle between the 2Fe2+2FeH state and the 3Fe2+FH state. Both cluster ligands are located near the amino-terminal end of the helices that are directed towards the cluster. NH-S bonds are form to provide stabilizing electrostatic forces to the center of the protein. Other than the cysteinyl ligands, there is little contact between the Fe4S4 cluster and other amono acid side chains. The image of the Fe protein cluster is that of an exposed and loosely packed center that acts as a pivot or hinge to allow any conformational change between subunits during the reaction of the enzyme. When the subunits pivot or swing together it is lowered towards the FeMo protein to ensure electron transfer as shown in the diagram below.[8] [9]
FeMo protein
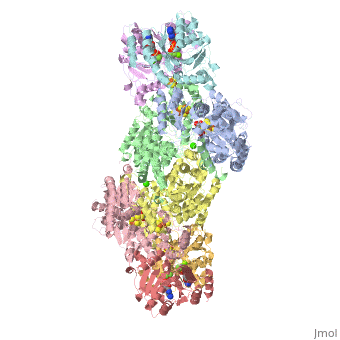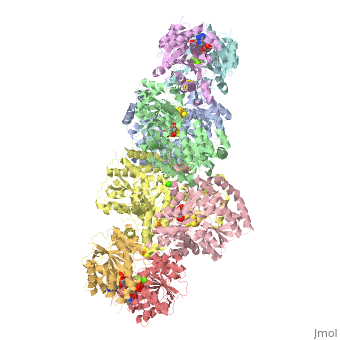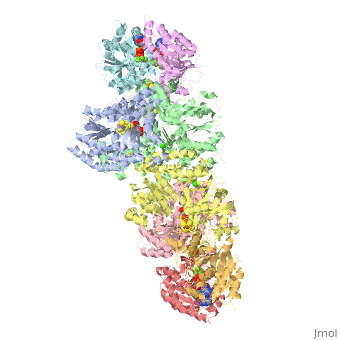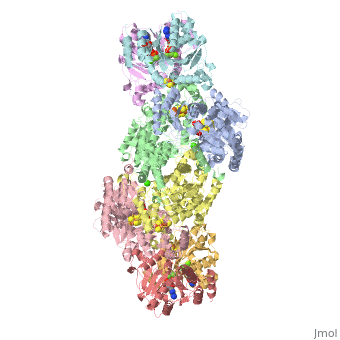
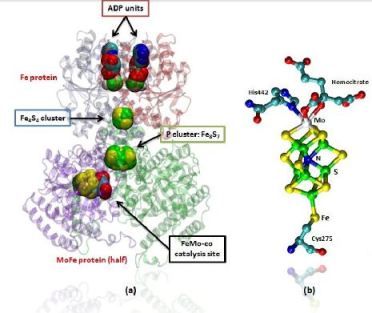
The MoFe-protein is a double α/β tetramer with a total molecular weight of about 240 kilo dalton.[10] The protein is known to posses two groups of metal centers: the P-cluster (which is located between each α and β interface) and the FeMo-cofactor or M-cluster (which is located in each of the α subunits)[11] as shown in diagram (a) below.
Nitrogenase ( FeMo protein and Fe protein) and FeMo-cofactor
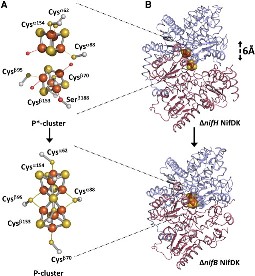
Each of the two FeMo-cofactor units contains 1 Molybdedum, 7 iron, 9 sulfur and one homocitrate molecule, and also serves as the site of substrate binding and subsequent reduction. The M-cluster is formed from Fe4S3 and MoFe3S3 partial cubanes that are joined together by three nonprotein ligands. The FeMo-cofactor is tucked -10 A beneath the protein surface, in an environment primarily provided by the α subunit. Only two protein ligands, Cys a275 and Hisa442, organize the cofactor to the protein. The octahedral ordered sphere of the Molybdenum is completed by the addition of homocitrate which together displaces a terminal Fe atom from the P-cluster. The remaining Fe and S are used to make up the P-cluster pairs. The role of the P-cluster is to transfer the electrons from the Fe-protein to the FeMo-cofactor or M-cluster.[12] It is connected to the backbone of the protein via 7 amino acid residues: six cysteines and one serine. Three cysteine residues are provided by α subunit while the rest come from the β subunit. There are two copies of the P-cluster in the MoFe protein which contain two Fe4S4 clusters that are reductively coupled and are now considered as a Fe8S8 cluster. What makes the P-cluster pair an unusual inorganic structure is that the two Fe4S4 clusters are connected by the thiol side chains of Cysteine α88 and β95, and a disulfide bond between two cluster inorganic sulfurs. This disulfide bond is located on the side of the P-cluster pair closest to the surface of the protein. The presence of the disulfide bond in the P-cluster pair shows that the center of the P-cluster serves as a two electron redox group. Since the Fe-protein is considered to be a one-electron donor with the P-cluster pair as the immediate acceptor, the P-cluster pair may be considered to be both.one- and two-electron redox reactant. [13]
NifH may act in a similar manner in assembly and catalysis, both of which involve the docking of NifH on NifDK, hence a conformational change occurs between the two proteins, and the electron transfer from the NifH to the NifDK; only in the case of P-cluster assembly, NifH is specifically required for the redox-tuning and the proper positioning of the Fe4S4-like clusters for efficient coupling. The two P-clusters in NifDK or the MoFe protein do not mature at the same time rather, they form one before the other with a gradual assembly of the αβ halves of the MoFe protein. [15]
MECHANISM
The nitrogenase mechanism is divided into two parts; the redox cycle between the Fe-protein and the MoFe-protein which provides electrons one by one, and the substrate reduction cycle which requires about eight electrons, depending on the amount of hydrogen produced.
- The redox cycle: the Fe-protein hydrolyzes MgATP and provides electrons to the MoFe-protein. The Fe-protein will be reduced by dithionite in vitro. In the cell, in vivo, the reduction will be done by electron carriers like NAD+, NADP+, or FAD2 for example, obtaining
The electrons used from the citric acid cycle or from glycolysis.[16] Two of the already bound MgADP molecules are replaced by new MgATP molecules via reduction. The next step is the complex formation between the Fe protein and the FeMo protein which requires the Fe-protein to be in its reduced state and bound to MgATP. Contraction of the proteins enables a conformational change and formation of the complex Fered(MgATP)2MoFe which is reversible. The hydrolysis of ATP occurs, therefore producing ADP and inorganic phosphate. One electron is transferred from the reduced Fe protein to the MoFe-protein. The order of these two processes is unknown but it has been proposed that it may occur simultaneously. The complex now changes to Feox(MgADP)2MoFered. The complex dissociates to form Feox(MgADP)2 + MoFered, which is believed to be the rate-limiting step for the whole substrate conversion. The rate of the complex dissociation is about 5s-1 under saturating conditions. The electron collected by MoFe-protein is used for substrate reduction and the Fe protein goes back to its starting point to end the redox or the Fe-protein cycle.
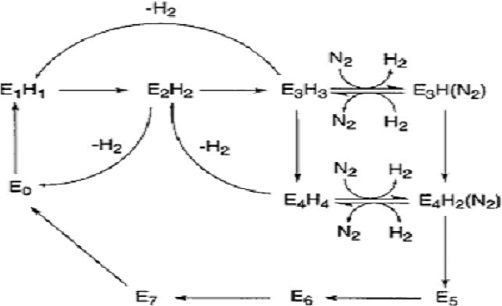
- The substrate reduction cycle: The substrate reduction cycle is not well known either but it is supposed that the Fe-protein cycle limits the electron transport to the active site of nitrogenase since it donates the electrons used in the substrate reduction. Thorneley & Lowe proposed a scheme for nitrogen fixation. Starting from the resting state E0 which represents the MoFe-protein at an oxidized state, one electron and proton are added to the protein which leads to the E1H1state as depicted in diagram. The notation ExHy describes the protein with x electrons and y protons added to the resting state. After the next electron-proton transfer step, reaching E2H2, there is a chance that the H2 production which would lead back to the resting state. This highly depends on the electron flux, that is, the concentration of the Fe-protein and its supply of the electrons needed compared to the concentration of the MoFe-protein. When there is a low flux, there is a low concentration of the Fe-protein compared to the MoFe-protein, the E1H1 state builds up H2 whereas the E2H2 is removed by evolving H2.[17] [18]
If flux of the Fe-protein is high, E2H2 will again be reduced and protonated, leading to E3H3, which again can produce H2. Dinitrogen then binds only when there are up to 3 or 4 electrons supplied.The binding of dinitrogen produces one H2 and leads to E3HN2. In its turn, H2 can act as an inhibitor to N2. The state E3H3 may be reduced to E4H4 which then can bind N2 along with H2 production. The protein goes back to its oxidized state if no more substrate is available to be reduced and N2 is protonated until NH3 is released.[19]
Since the FeMo-protein undergoes its major changes at the binding
region of the Fe-protein during complex formation, it is understood that the substrates enter and leave protein through the homocitrate – P-cluster pathway.[20]
[21]
Reactions with the nitrogenase system include the following;
2H+ + 2e- →H2
N2 + 8H+ + 8e- →2NH3 + H2
N2O + 2H+ + 2e- + N2 + H2O
CN- + 7H+ + 6e- →CH4 + NH3
CH3NC + 6+ + 6e- →CH3NH2 + CH4
N3- + 3H+ + 2e- → N2 + NH3
CH2H2 + 2H+ + 2e- →CH2H2
CH2CH=CH +2H+ + 2e- →CH2CH2CH2
H2N-CN + 6H+ + 6e- → NH3 + CH3NH2
CH2 N=N + 6H' + 6e- → CH3NH2 + NH3
All substrates in the reactions above compete for electrons from the same pool of reduced dinitrogenase, as they inhibit each other. [22] Among the substrates listed, only N2O is a competitive inhibitor of N2 reduction, and the others are noncompetitive inhibitors. Other inhibitors include CO, H2 , CS2.[23]
APPLICATION OF DEHYDROGINASE
Nitrogen is abundant and makes up about 70% of the atmosphere. It is required by plants as fertilizer (nutrient), but they can not readily access it because dinitrogen as we have discussed earlier is bound by one of the strongest covalent bonds in nature (triple bond). Industries use the Haber-Bosch process, a method developed by Fritz Haber and Carl Bosch to break this bond and convert dinitrogen to ammonia, requiring about 500 and 450 bar. Around 80·109 kg of ammonia are manufactured every year through this process. Nature, on the other hand converts dinitrogen to ammonia in anerobic environments and low temperature conditions through the use of nitrogenase. Biological nitrogen fixation produces two or three times more ammonia per year than the industrial process. [24]
REFERENCES
- ↑ Rubio, L. M., Ludden, P. W., Maturation of Nitrogenase:a biochemical puzzle. (2005) J. Bacteriol. 187, 404-414.
- ↑ Burris, R. H., Nitrogenases. (1991) J. Biol. Chem. 266,9339-9342.
- ↑ Burris, R. H., Nitrogenases. (1991) J. Biol. Chem. 266,9339-9342.
- ↑ Jian-Feng Z., Hui-Na Z., Ying Z., Shao-Min B., Ju-Fu H. Advances in Studies on Nitrogenase Crystallography.(2004) The Chinese Academy of Science.46, 392-400.
- ↑ Jian-Feng Z., Hui-Na Z., Ying Z., Shao-Min B., Ju-Fu H. Advances in Studies on Nitrogenase Crystallography.(2004) The Chinese Academy of Science.46, 392-400.
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Ress D.C., Howard J.B. NITROGENASE: A Nucleotide-Dependent Molecular Switch.(1994) Annu. Rev. Biochem. 63, 235-264.
- ↑ Ress D.C., Howard J.B. NITROGENASE: A Nucleotide-Dependent Molecular Switch.(1994) Annu. Rev. Biochem. 63, 235-264.
- ↑ Hu, Y., Ribbe, M. W., Nitrogenase Assembly. (2013) Biochemica et Biophysica Acta. 1827,1112-1122.
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Thorneley R.N.F., Lowe D.J. Nitrogenase: substrate binding and activation.(1996) JBIC.1, 576-580.
- ↑ http://www.chem.utoronto.ca/coursenotes/GTM/JM/N2start.htm
- ↑ Ress D.C., Howard J.B. NITROGENASE: A Nucleotide-Dependent Molecular Switch.(1994) Annu. Rev. Biochem. 63, 235-264.
- ↑ Hu, Y., Ribbe, M. W., Nitrogenase Assembly. (2013) Biochemica et Biophysica Acta. 1827,1112-1122.
- ↑ Hu, Y., Ribbe, M. W., Nitrogenase Assembly. (2013) Biochemica et Biophysica Acta. 1827,1112-1122.
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Thorneley R.N.F., Lowe D.J. Nitrogenase: substrate binding and activation.(1996) JBIC.1, 576-580.
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
- ↑ Hu, Y., Ribbe, M. W., Nitrogenase Assembly. (2013) Biochemica et Biophysica Acta. 1827,1112-1122.
- ↑ Burris, R. H., Nitrogenases. (1991) J. Biol. Chem. 266,9339-9342.
- ↑ Rivera-ortiz J.M., Burris R.H. Interactions Among Substrates and Inhibitors of Nitrogenase. (1975) J. Bacteriol. 123, 537-545.
- ↑ Schimpl J. K. Biological Nitrogen Fixation –Simulation of the Reaction Mechanism of Nitrogenase from First Principles. (2004).
"RCSB Protein Data Bank - RCSB PDB - 1N2C Structure Summary." RCSB Protein Data Bank - RCSB PDB - 1N2C Structure Summary. N.p., n.d. Web. 03 Dec. 2013