We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 765
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
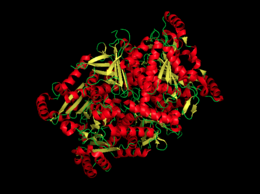
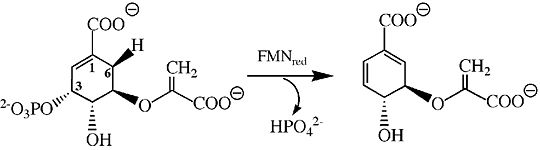
Chorismate Synthase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Crystal structure of Chorismate synthase complexed with oxidized FMN and EPSP." RSCB Protein Data Bank. RCSB. Web. 30 Nov. 2013. http://www.rcsb.org/pdb/explore/explore.do?structureId=1qxo.
- ↑ Marchenko LF, Arsen'eva EN, Ignatiuk TE, Semenova EI, Sapelkina LV. [Hydroxyproline and somatotropic hormone in children of mothers with diabetes mellitus]. Pediatriia. 1987;(7):11-3. PMID:3670998
- ↑ Arcuri HA, Palma MS. Understanding the structure, activity and inhibition of chorismate synthase from Mycobacterium tuberculosis. Curr Med Chem. 2011;18(9):1311-7. PMID:21366532
- ↑ Fitzpatrick TB, Killer P, Thomas RM, Jelesarov I, Amrhein N, Macheroux P. Chorismate synthase from the hyperthermophile Thermotoga maritima combines thermostability and increased rigidity with catalytic and spectral properties similar to mesophilic counterparts. J Biol Chem. 2001 May 25;276(21):18052-9. Epub 2001 Mar 9. PMID:11279147 doi:http://dx.doi.org/10.1074/jbc.M100867200
- ↑ Macheroux P, Schmid J, Amrhein N, Schaller A. A unique reaction in a common pathway: mechanism and function of chorismate synthase in the shikimate pathway. Planta. 1999 Jan;207(3):325-34. PMID:9951731
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Kitzing K, Auweter S, Amrhein N, Macheroux P. Mechanism of chorismate synthase. Role of the two invariant histidine residues in the active site. J Biol Chem. 2004 Mar 5;279(10):9451-61. Epub 2003 Dec 10. PMID:14668332 doi:http://dx.doi.org/10.1074/jbc.M312471200
- ↑ Osborne A, Thorneley RN, Abell C, Bornemann S. Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction. J Biol Chem. 2000 Nov 17;275(46):35825-30. PMID:10956653 doi:http://dx.doi.org/10.1074/jbc.M005796200
- ↑ Osborne A, Thorneley RN, Abell C, Bornemann S. Studies with substrate and cofactor analogues provide evidence for a radical mechanism in the chorismate synthase reaction. J Biol Chem. 2000 Nov 17;275(46):35825-30. PMID:10956653 doi:http://dx.doi.org/10.1074/jbc.M005796200
- ↑ Floss HG, Onderka DK, Carroll M. Stereochemistry of the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthetase reaction and the chorismate synthetase reaction. J Biol Chem. 1972 Feb 10;247(3):736-44. PMID:4550759