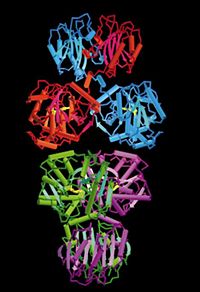Sandbox Reserved 767
From Proteopedia
|
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Contents |
Introduction
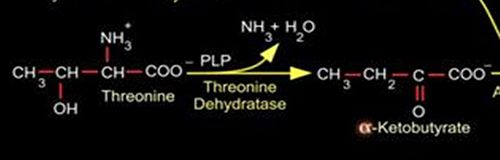
Threonine dehydratase (TDH) also known as Threonine Ammonia-Lyase and Threonine Deaminase is a tetrameric enzyme of about 200kD that catalyzes the dehydration of threonine to α-ketobutyrate. TDH is the first enzyme in the isoleucine biosynthetic pathway. It belongs to the enzyme class EC-4 which cleaves C-C, C-O, C-N through hydrolysis or oxidation. TDH EC 4.3.1.19 specifically cleaves C-N bonds. The enzyme was first discovered in E.coli around 1965 but has since been purified from other bacterial sources such as Salmonella typhimurium. TDH exists in two forms in bacteria: biosynthetic threonine dehydratase (BTD) and catabolic threonine dehydratase (CTD). While BTD catalyzes the first reaction in the biosynthetic pathway of isoleucine in an aerobic environment, CTD functions in the degradation of threonine to propionate in anaerobic conditions. The feedback inhibition of TDH in E.coli served as a model for metabolic regulation as it was one of the earliest examples observed.
Structure
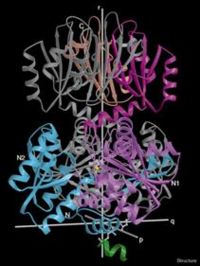
Threonine deaminase, purified from Salmonella typhimurium has a molecular weight of about 200kD which exists as tetramer in its native state and consists of four identical subunits. In the absence of the cofactor pyridoxal phosphate, TDH dissociates from its tetrameric form to about 2 individual 100 kD dimers. It is 514 residues in length with a secondary structure that is 37% helical and 17% beta sheet.From its quaternary structure, the prolate ellipsoidal shape of TDH can be observed. There are 3 perpendicular axes that intersect at the center. These arbitrary axes (p,q,r) can be viewed in Fig. 1 . The core of the tetramer is composed of the four N-terminal catalytic domains while the C-terminal regulatory domains which lie across the r axis form the ends of the tetramer. As can be seen from the Figure 1, each subunit interacts individually with two other subunits.
Protein Fold and Organization of Domain
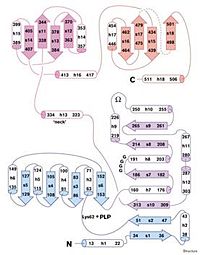
The catalytic domain, N-terminal domain, possesses the cofactor of TDH. This domain includes residues 1-320 and is shown in blue and purple and is comprised of parallel beta sheets(arrows) with flanking helices(cylinders). The regulatory domain, the C-terminal is shown as magenta and purple and consists of residues 321-514. This portion folds as an antiparallel sheet with helices on the side. This domain can be divided into 2 similar sheets with the helices facing the outside and the sheets closer to the core. The missing zone, residues 481-496, represent the exterior of the domain.
The two domains are connected by a neck region, residues 323-334 which is the main contact between both regions. This neck region has a right- handed twist that results in the adjacent relationship of the catalytic to the regulatory domains.Regulation of TDH
TDH, the first enzyme in the biosynthetic pathway of Isoleucine is feedback inhibited by the end product of this pathway, isoleucine. When there is ample isoleucine in the cell, to prevent accumulation of the amino acid, isoleucine allosterically binds to TDH, therefore preventing the conversion of threonine to α-ketobutyrate.
Mechanism of Action of TDH
Cofactor
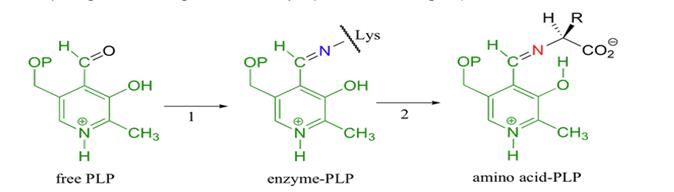
For TDH to be catalytically active, pyridoxal phosphate (PLP), its prosthetic cofactor must be attached to it. This is initiated by the formation of a Schiff base linkage between the aldehyde group of PLP and the Lys-62 residue of TDH. The electrophilic nitrogen of the pyridine ring of PLP acts as an electron sink where excess electrons can be delocalized. This effect draws electrons from TDH thereby stabilizing the carbanion intermediate. PLP is wedged between the two subdomains of the catalytic domain close to the N-terminal. Below is an illustration of the mechanism of attachment of the cofactor PLP a lysine residue. As can be seen, the Schiff base is formed between PLP and the lysine residue.
Deamination
Once the cofactor, PLP attaches to TDH, the complex is catalytically active. The reaction proceeds by the removal of ammonia from threonine which produces a keto-acid intermediate. Dehydration also takes place as part of the reaction.
Biological Implication
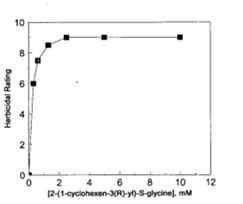
Enzymes involved in amino acid biosynthetic pathways are common targets of herbicides. An antimetabolite 2-(1-cyclohexen-3(R)-yl)-S-glycine CHG is known to inhibit the growth of a plant Arabidopsis thaliana by inhibiting the action of TDH on the pathway. When CHG was added to the plant, the effect of its increasing amount recorded and can be seen in Figure 4 . From the figure below, it takes a little increase of CHG to go from no herbicidal effect to total herbicidal rating which corresponds to death of the plant. Interestingly, the herbicidal effect CHG can be reversed by isoleucine or some of the intermediate of its biosynthetic pathway.This observation emphasizes the importance of TDH in plant cells which when inhibited by CHG can alter plant growth. This characterizes TDH as a suitable target in herbicidal ingredients.
References
- "Crystal Structures of Salmonella Typhimurium Biodegradative Threonine Deaminase and Its Complex with CMP Provide Structural Insights into Ligand-induced Oligomerization and Enzyme Activation*." Crystal Structures of Salmonella Typhimurium Biodegradative Threonine Deaminase and Its Complex with CMP Provide Structural Insights into Ligand-induced Oligomerization and Enzyme Activation. N.p., n.d. Web. <http://www.jbc.org/content/281/51/39630>.
- "RCSB Protein Data Bank - RCSB PDB - 1TDJ Annotations Report." RCSB Protein Data Bank - RCSB PDB - 1TDJ Annotations Report. N.p., n.d. <http://www.rcsb.org/pdb/explore/derivedData.do?structureId=1TDJ>.
- "Study and Reengineering of the Binding Sites and Allosteric Regulation of Biosynthetic Threonine Deaminase by Isoleucine and Valine in Escherichia Coli - Springer." Study and Reengineering of the Binding Sites and Allosteric Regulation of Biosynthetic Threonine Deaminase by Isoleucine and Valine in Escherichia Coli - Springer. N.p., 01 Apr. 2013. Web. <http://link.springer.com/article/10.1007/s00253-012-4176-z>.
- "Threonine Ammonia-lyase." Wikipedia. Wikimedia Foundation, 28 Oct. 2013. Web. <http://en.wikipedia.org/wiki/Threonine_ammonia-lyase>.
- "Inhibition of Threonine Dehydratase Is Herbicidal." Inhibition of Threonine Dehydratase Is Herbicidal. N.p., n.d. Web. <http://www.plantphysiol.org/content/106/4/1257.long>.