Sandbox Reserved 771
From Proteopedia
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
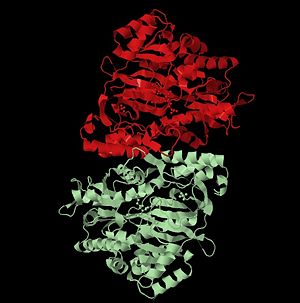
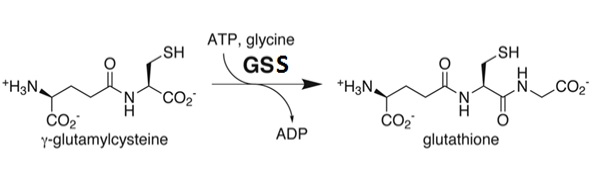
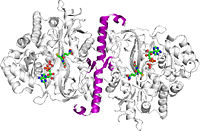
Glutathione synthetase (GSS) is an homo-dimeric, ATP-depending ligase responsible for the condensation of γ-Glutamylcysteine and glycine to form Glutathione (GSH) during the second step of the glutathione biosynthesis pathway [1]. Glutathione is considered to be one of the most abundant and important antioxidants present across many bacterial (cyano- and proteobacteria), and all plant & mammalian cells [2]. In addition to protecting cells from the oxidative damage caused by free radicals, it is believed to be involved in the detoxification of xenobiotics, toxins in the blood, and even amino acid transport [3].
Contents |
Reaction Mechanism
Glutathione Synthetase is the key enzyme involved in the ATP-dependent condensation of γ-Glutamylcysteine and glycine to form Glutathione during the second step of the glutathione biosynthesis pathway [4]. [5]. The condensation begins by binding of ATP to GSS in the presence of γ-Glutamylcysteine, to form an enzyme-bound acyl-phosphate that binds glycine and generates the enzyme-product complex. Dissociation of GSS from the E::P complex results in release of GSH, ADP, and inorganic phosphate (Pi) [6]. The ATP-dependence of the catalysis qualifies GSS for inclusion into the ligase enzyme superfamily. Further, a Hill constant of ~0.67 indicates that GSS exhibits negative cooperativity towards the substrate γ-Glutamylcysteine [7].
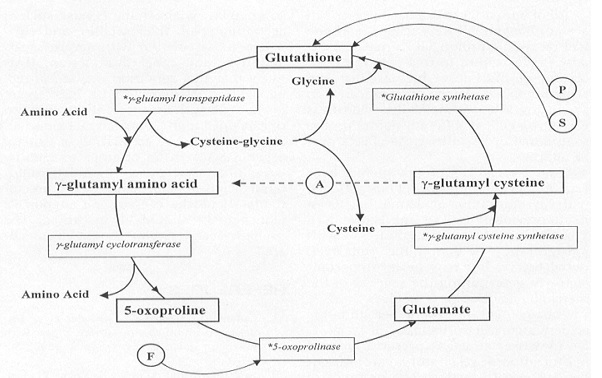
The glutathione biosynthesis pathway is an inter-dependent cycle, exhibiting a regulatory ability through the negative cooperativity of the second step of the cycle -- the step catalyzed by GSS [8]. Below, you can see the full cycle including the substrates, cofactors, and enzymes involved in each step of the reaction.
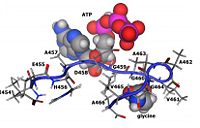
Substrate and ATP Binding Residues
| |||||||||||
Alternative Splicing Variants [12]
While the expression of glutathione synthetase (GSS) has been studied and fairly well characterized, the sequence and alternative splicing of the gss gene has been studied very little. Using real-time polymerase chain reaction (qPCR) to quantify mRNA levels of the gss transcript within human cells has revealed one common alternative splicing variant present within colon, kidney, lung, liver, placenta, blood, and uterus cells. It has not, however, been detected within heart, skeletal muscle, and spleen tissue cells. This ASV is produced from a 333 bp in-frame deletion, including the complete removal of exons 4 and 5.
Glutathione Deficiency Syndrome [13]
Though reduced levels of GSH have been observed in patients with Alzheimers and Parkinsons, inborn errors in the endogenous GSS enzyme resulting in significantly low levels of GSH is believed to be the cause of a very rare metabolic deficiency in which there is a large build up of 5-oxoproline in the urine - termed 5-oxoprolinuria. It is so rare that, as of 2006, it had been diagnosed in less than 100 people worldwide.
GSH deficiency has been sub-divided into three forms: Mild, Moderate, and Severe. Mild deficiencies usually result in what is known as hemolytic anemia, and is a result of the degradation of red blood cells. Rarely, mild GSH deficiencies can result in 5-oxoprolinuria. More moderate GSS deficiencies will result in a higher likelihood of developing 5-oxoprolinuria, hemolytic anemia, and metabolic acidosis - a condition in which the blood pH is lower than the homeostatic pH of 7 - shortly after birth. Finally, individuals with severe GSH deficiencies experience severe neurological symptoms in addition to those associated with moderate GSH deficiencies. Slowed physical movements, reactions, and speech, as well as intellectual retardation and a loss of coordination are those frequently associated with severe GSH deficiency.
Studies suggest that the rarity of this disorder can be attributed to the fact it is autosomal recessive and thus both copies of the cell's chromosome must contain the genetic coding for the disorder. Each of the parents must carry a single copy of the mutated gss gene, thus displaying no physical symptoms, and both must pass their mutated copy on to the child.
References
- ↑ Uchida M, Sugaya M, Kanamaru T, Hisatomi H. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep. 2010 Apr;37(4):2105-9. doi: 10.1007/s11033-009-9675-3. Epub 2009, Aug 12. PMID:19672693 doi:http://dx.doi.org/10.1007/s11033-009-9675-3
- ↑ http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
- ↑ Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem Biophys Res Commun. 2011 Jul 8;410(3):597-601. doi:, 10.1016/j.bbrc.2011.06.034. Epub 2011 Jun 12. PMID:21683691 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.034
- ↑ http://www.ncbi.nlm.nih.gov/protein/NP_000169.1
- ↑ 21771585
- ↑ Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. The role of the glycine triad in human glutathione synthetase. Biochem Biophys Res Commun. 2010 Oct 1;400(4):511-6. doi:, 10.1016/j.bbrc.2010.08.081. Epub 2010 Aug 26. PMID:20800579 doi:http://dx.doi.org/10.1016/j.bbrc.2010.08.081
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Brown TR, Drummond ML, Barelier S, Crutchfield AS, Dinescu A, Slavens KD, Cundari TR, Anderson ME. Aspartate 458 of human glutathione synthetase is important for cooperativity and active site structure. Biochem Biophys Res Commun. 2011 Aug 5;411(3):536-42. doi:, 10.1016/j.bbrc.2011.06.166. Epub 2011 Jul 12. PMID:21771585 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.166
- ↑ Slavens KD, Brown TR, Barakat KA, Cundari TR, Anderson ME. Valine 44 and valine 45 of human glutathione synthetase are key for subunit stability and negative cooperativity. Biochem Biophys Res Commun. 2011 Jul 8;410(3):597-601. doi:, 10.1016/j.bbrc.2011.06.034. Epub 2011 Jun 12. PMID:21683691 doi:http://dx.doi.org/10.1016/j.bbrc.2011.06.034
- ↑ Dinescu A, Brown TR, Barelier S, Cundari TR, Anderson ME. The role of the glycine triad in human glutathione synthetase. Biochem Biophys Res Commun. 2010 Oct 1;400(4):511-6. doi:, 10.1016/j.bbrc.2010.08.081. Epub 2010 Aug 26. PMID:20800579 doi:http://dx.doi.org/10.1016/j.bbrc.2010.08.081
- ↑ Uchida M, Sugaya M, Kanamaru T, Hisatomi H. Alternative RNA splicing in expression of the glutathione synthetase gene in human cells. Mol Biol Rep. 2010 Apr;37(4):2105-9. doi: 10.1007/s11033-009-9675-3. Epub 2009, Aug 12. PMID:19672693 doi:http://dx.doi.org/10.1007/s11033-009-9675-3
- ↑ PMID:10369661</red>. <ref>http://ghr.nlm.nih.gov/condition/glutathione-synthetase-deficiency</li></ol></ref>