Sandbox Reserved 775
From Proteopedia
|
| This Sandbox is Reserved from Sep 25, 2013, through Mar 31, 2014 for use in the course "BCH455/555 Proteins and Molecular Mechanisms" taught by Michael B. Goshe at the North Carolina State University. This reservation includes Sandbox Reserved 299, Sandbox Reserved 300 and Sandbox Reserved 760 through Sandbox Reserved 779. |
To get started:
More help: Help:Editing |
Contents |
Introduction
Nisin is a broad spectrum bacteriocin produced by the bacterium Lactococcus lactis. It is currently used in the food industry as a natural preservative (Burrowes and others 2004). It is often used as an antimicrobial agent in processed cheese to prevent the growth of various Clostridium bacteria that were present in the initial ingredients that survived the heat treatment of the cheese. In addition to processed cheese, Nisin is used in many other food products such as salad dressing, alcoholic beverages, canned vegetables, and meat (Delves-Broughton 2005).
Bacteriocins are antimicrobial peptides produced by bacteria to inhibit other closely related strains. These chemicals are distinguished from antibiotics in various ways. Bacteriocins are synthesized in the ribosome with a narrow activity spectrum while antibiotics are secondary metabolites and their spectrums vary. Bacteriocins do not harm the host cell and, unlike antibiotics, have no known toxicity to humans. Nisin is the only FDA approved bacteriocin currently used in food (Burrowes and others 2004). Unusually, it has a broad spectrum of activity, as it inhibits many gram positive bacteria. While nisin is effective against many gram positive bacteria, it is ineffective at inhibiting gram negative strains (Delves-Broughton 2005).
Structure
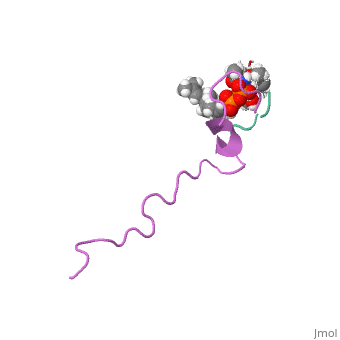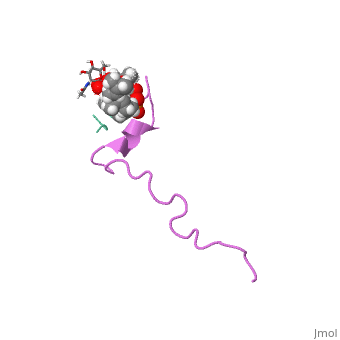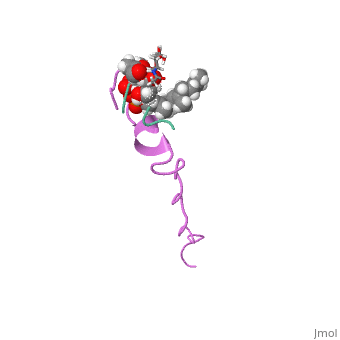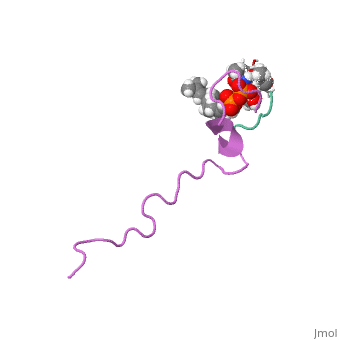
Nisin has a molecular weight of 4695.47 Da and contains 34 residues. It contains many cyclic structures composed of unusual amino acid residues that are post translationally modified such as lanthionine, 3-methyl-lanthionine, dehydroaline, and dehydrobutyrine (Chan and others 1996; Fukao and others 2008). Nearly 80% of the amino acid residues are found in these cyclic rings. Consequently, this peptide exhibits very little secondary structure (van den Hooven and others 1993). Most shifts in NMR spectra happen at the N-terminus of the peptide which further supports this fact(van den Hooven and others 1993).The structure of this peptide is not very well defined. It is known that once Nisin moves from an aqueous environment to a hydrophobic one, the secondary structure changes(van den Hooven and others 1993). Infrared spectroscopy shows that this peptide either has an alpha helical structure or is a random coil in aqueous environments(El-Jastimi and Lafleur 1997). When dissolved in heavy water, it is clear that Nisin largely does not have an ordered structure and has beta turns(El-Jastimi and Lafleur 1997). It is also known that this peptide contains 2 thioether linkages that are critical for binding to the bacterial membrane(Fukao and others 2008). NMR spectroscopy has shown that nisin is monomeric in nature as all NOE’s were intermolecular(van den Hooven and others 1993). The NMR spectra differs considerably when nisin is dissolved in aqueous environments when compared to hydrophobic environments. The use of different solvents also cause different circular dichroism spectra to form as well(van den Hooven and others 1993). Such data suggests that the conformation of nisin changes depending on the hydrophobicity of the environment.
Mode of Action
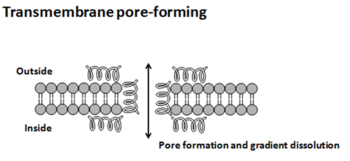
This peptide inhibits bacteria by creating pores in the cytoplasmic membrane, which disrupts the proton motive force. Because it has a +5 charge, it is thought that Nisin electrostatically binds to the negatively charged bacterial membrane(El-Jastimi and Lafleur 1997). It has been found that Nisin has a greater affinity to negatively charged lipids than zwitterionic lipids(El-Jastimi and Lafleur 1997). There are three proposed models of inhibition: barrel-stave, torroidal, and carpet (Bechinger and Lohner 2006). For the barrel-stave and torroidal models, it is thought that the positively charged peptide is electrostatically attracted to the negatively charged cytoplasmic membrane. Once the peptide comes into contact with the membrane, the peptide folds itself into an alpha helical structure, and when a high enough concentration of peptides adsorbs onto the membrane surface, a pore forms in the following manner. In the barrel-stave model, the peptides form a very ordered barrel like structure through the membrane creating a pore that intercellular fluids can leak out of (Bechinger and Lohner 2006). In the torroidal pore model, pore formation occurs either through the AMP imbedding itself into the membrane or through positive curvature strain (Bechinger and Lohner 2006). The pores formed through either of these models can cause leaking of intercellular fluid and the loss of membrane potential. In the carpet model, AMP’s aggregate onto the bacterial membrane much like the barrel stave and the torroidal model; but instead of imbedding themselves into the membrane; the peptides form a carpet like structure on the outer surface of the membrane by electrostatically binding to the phospholipid head group and directly dissolving the membrane (Oren and Shai 1998). The structure causes the membrane to break into pieces through micellization (Bechinger and Lohner 2006). Non-membrane targeting AMP’s pass through the bacterial membrane without damaging it and have been observed inhibiting protein synthesis, nucleic acid synthesis, enzymatic activity, and cell wall synthesis (Zhang and others 2001).
Lipid II appeared to be an important molecule in the process of binding Nisin to the bacterial membrane. Lipid II is a membrane-bound precursor used for the synthesis of peptidoglycan for the bacterial cell wall(Hsu and others 2004). Nisin specifically binds with Lipid II which in turn increases the antimicrobial efficacy of the peptide. However, the exact mechanism of binding between Nisin and Lipid II is still unknown(Hsu and others 2004).
References
Bechinger B, Lohner K. 2006. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochimica Et Biophysica Acta-Biomembranes 1758(9):
Burrowes O, Hadjicharalambous C, Diamond G, Lee T. 2004. Evaluation of antimicrobial spectrum and cytotoxic activity of pleurocidin for food applications. J.Food Sci. 69(3):M66-71.
Chan WC, Leyland M, Clark J, Dodd HM, Lian L-, Gasson MJ, Bycroft BW, Roberts GCK. 1996. Structure-activity relationships in the peptide antibiotic nisin: antibacterial activity of fragments of nisin. FEBS Lett. 390(2):129-32.
Delves-Broughton J. 2005. Nisin as a food preservative. Food Aust. 57(12):525-7.
El-Jastimi R, Lafleur M. 1997. Structural characterization of free and membrane-bound nisin by infrared spectroscopy. Biochimica et Biophysica Acta (BBA) - Biomembranes 1324(1):151-8.
Fukao M, Obita T, Yoneyama F, Kohda D, Zendo T, Nakayama J, Sonomoto K. 2008. Complete covalent structure of nisin Q, new natural nisin variant, containing post-translationally modified amino acids. Biosci.Biotechnol.Biochem. 72(7):1750-5.
Hsu ST, Breukink E, Tischenko E, Lutters MA, de Kruijff B, Kaptein R, Bonvin AM, van Nuland NA. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat.Struct.Mol.Biol. 11(10):963-7.
Oren Z, Shai Y. 1998. Mode of action of linear amphipathic ?-helical antimicrobial peptides. Peptide Science 47(6):451-63.
van den Hooven HW, Fogolari F, Rollema HS, Konings RNH, Hilbers CW, van de Ven FJM. 1993. NMR and circular dichroism studies of the lantibiotic nisin in non-aqueous environments. FEBS Lett. 319(1–2):189-94.
Zhang LJ, Rozek A, Hancock REW. 2001. Interaction of cationic antimicrobial peptides with model membranes. J.Biol.Chem. 276(38):