This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 921
From Proteopedia
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
Fatty Acid Amide Hydrolase
Introduction
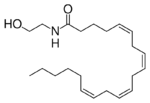
Fatty acid amide hydrolase (FAAH) is the primary catabolic enzyme for the degradation of fatty acid amides. FAAH primarily degrades anandamide, which is an endocannabinoid that activates the CB1 and CB2 cannabinoid receptors. When CB1 and CB2 cannabinoid receptors are active, the receptors affect appetite, sleep, and relief of pain. The ability to inhibit FAAH has been widely investigated for possible pain relief medication.
An inhibitor binds to the active site of the enzyme lowering the rate at which the enzyme degrades substrate, thus enzyme inhibitors are often used as drugs. A FAAH inhibitor offers great potential as a pain relief drug because FAAH commonly degrades anandamide. If the enzymatic activity of FAAH was lowered there would be increased levels of anandamide causing increased appetite, sleep, and pain relief.
A recent study on FAAH inhibitors combined an irreversible bond at Cys269 and a reversible bond at Ser241 of the active site.[1] A humanized rat variant of FAAH was inhibited by BR1, CL, and PEG. The mice displayed an increase in endogenous brain levels of the FAAH substrate anandamide for over six hours.[1] This is the first step towards developing a long lasting pain relief medication by inhibiting FAAH.
| |||||||||||
References
- ↑ 1.0 1.1 Otrubova K, Brown M, McCormick MS, Han GW, O'Neal ST, Cravatt BF, Stevens RC, Lichtman AH, Boger DL. Rational design of Fatty Acid amide hydrolase inhibitors that act by covalently bonding to two active site residues. J Am Chem Soc. 2013 Apr 24;135(16):6289-99. doi: 10.1021/ja4014997. Epub 2013 Apr, 12. PMID:23581831 doi:http://dx.doi.org/10.1021/ja4014997
- ↑ http://en.wikipedia.org/wiki/FAAH
- ↑ Kono M, Matsumoto T, Kawamura T, Nishimura A, Kiyota Y, Oki H, Miyazaki J, Igaki S, Behnke CA, Shimojo M, Kori M. Synthesis, SAR study, and biological evaluation of a series of piperazine ureas as fatty acid amide hydrolase (FAAH) inhibitors. Bioorg Med Chem. 2013 Jan 1;21(1):28-41. doi: 10.1016/j.bmc.2012.11.006. Epub, 2012 Nov 15. PMID:23218778 doi:http://dx.doi.org/10.1016/j.bmc.2012.11.006
- ↑ Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002 Nov 29;298(5599):1793-6. PMID:12459591 doi:10.1126/science.1076535