Sandbox Reserved 935
From Proteopedia
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Contents |
BRI865-1196ADP (4OA2)
|
Introduction
Brassinosteroid insensitive 1 (BRI1) is a membrane receptor that senses brassinosteroides, which are polyhydroxylated steroid hormones [1]. Brassinosteroids control plant growth and development by activating BRI1 and starting a signaling pathway that leads to nuclear transcription factors being activated. They do so by inducing rapid plant cell wall expansion and hyperpolarization [2]. The brassinosteroid induced association of BRI1 with the proton pump of P-type H+-ATPase also seems to be necessary for the activation of P-ATPase in the plasma membrane, though it is not currently known if it’s by serine/threonine phosphorylation or some other protein-protein interaction. Both activation of the kinase domain and activation of the P-ATPase are necessary for the induction of the hyperpolarization and cell-wall expansion and brassinolides affect the interaction between them [3]. BRI1 is thought to be a dual-specificity kinase and it has structural features reminiscent of both serine/threonine and tyrosine kinases [1].
In the cell, BRI1 cycles between the plasma membrane and endosomes [1]. When not activated, BRI1 is auto-inhibited by its own C-terminal tail as well as auto-phosphorylation of Tyrosine-872 and interaction with BRI1 kinase inhibitor protein BKI1 in the kinase domain. BKI1 also contains an N-terminal targeting motif for the plasma membrane. When the extracellular LRR domain gets activated by a brassinosteroid, it causes a reordering of 70 residues and creates a docking platform for the co-receptor SERK3 (somatic embryogenesis receptor kinase 3, also known as BAK1, BRI1-associated receptor kinase 1). The C-termini of BRI1 and SERK3 trans-phosphorylate each other, releasing the BKI1 from BRI1 and allowing BRI1 to phosphorylate immediate downstream signaling components (such as BRI1 substrate kinases, also known as BSKs).
In this article we will concentrate on the BRI1 kinase domain and its interaction with the nucleotides ATP/ADP. Pictured here is the .
Structure
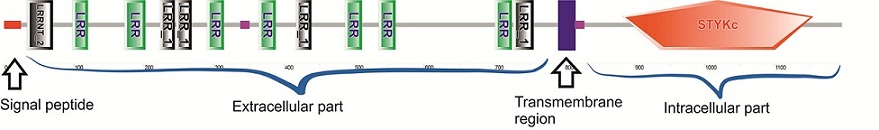
BRI1 belongs to a subgroup of the plant receptor-like kinases (RLKs) which are transmembrane receptor kinases. Plants have large numbers of RLKs (e.g. in Arabidopsis about 2.5% of all annotated protein coding genes belong to RLKs, rice possesses a similar number of RLKs) [4]. RLKs typically possess an intracellular kinase domain, a transmembrane region, extracellular domain(s) and a signal peptide. RLKs have been divided into 45 subfamilies based on their extracellular domains. The extracellular domain of BRI1 contains several leucine-rich repeat domains and thereby belongs to leucine-rich repeat-receptor-like kinases (LRR-RKs) . LRR-RKs are by far the largest group of RLKs in Arabidopsis [1]. The BRI1 nucleotide binding site is located between the N- and C-lobes of the protein.
The kinase domain adopts an active conformation with a salt-bridge between Lysine-911 and Glutamate-927 [1]. There is also a hydrogen bond between Glutamate-927 and Tyrosine-956. This tyrosine residue is a gatekeeper determining the size of the nucleotide binding pocket. Comparison with other plant receptor-like kinases suggests this hydrogen bond interaction and salt-bridge are important for the activation, because they are holding the binding pocket in its active conformation.
Function
BRI1 is a dual-specificity kinase: it has serine/threonine kinase activity and it can both autophosphorylate on tyrosine residues in the kinase and juxtamembrabe domains (residues 814-865, part of which is not in this model) and also transphosphorylate tyrosine residues in other proteins [1]. The juxtamembrane domain is important for the kinase domain activity.
In this structure, the adenine and ribose parts of ADP are well ordered, whereas the diphosphate is more flexible [1]. There are hydrogen-bonding interactions of the adenine base and ribose with the BRI1 hinge region main chain atoms (Glutamate-957, Methionine-959) and with two water molecules. When it was an ATP, the catalytic Aspartate-1009 caused it to γ -phosphate to face outwards. BRI865-1196 can efficiently hydrolyse ATP to ADP and to a lesser extent GTP to GDP.
There is a balance between phosphorylation dependent activation and several potential mechanisms for deactivation of BRI1 [5]. One of them involves autophosphorylation of serine891 in the ATP binding domain, which is involved in the binding and positioning of ATP. The phosphorylation of this residue reduces, but doesn't completely inhibit, the activity of the kinase domain. When this serine is mutated into aspartate or glutamate (which are residues with negative charge and probably function as phosphomimetics), it results into dwarfed Arabidopsis plants. Phosphorylation of Serine-891 also reduces autophosphorylation on several other residues that are phosphorylated after brassinosteroid induced activation. The deactivation associated with serine891 phosphorylation seems to play a role in attenuation of signaling rather than maintaining the inactive state of BRI1 in the absence of its hormone ligand.
Threonine-872 in the juxtamembrane domain is another phosphorylation site [5]. Its phosphorylation may inhibit the kinase activity and follows soon after the the brassinosteroid activation of the domain. Tyrosine-831 in the juxtamembrane domain is not essential for the activity of the kinase domain, but it does have a regulatory role [6]. Its phosphorylation causes inhibition of plant growth and delay of flowering. On the other hand, Tyrosine-956 in the kinase domain is important for kinase activity and its phosphorylation is believed to have an inhibitory role.
Homologs
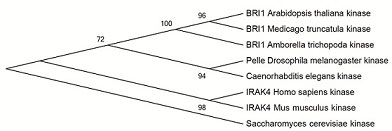
There are homologs for the kinase domain of BRI1 not only in plants but also in other eukaryotes [1]. Homologs in plants have similar domain structure in the whole protein, but in animals the other domains than kinase domain are different. The closest similarity to the kinase domain of BRI1 in Arabidopsis thaliana is interleukin-1 receptor-associated kinase 4 (IRAK4) in human and mouse. Similar kinase in Drosophila melanogaster is called Pelle-kinase. Structural similarity between kinase domains of BRI1 and human IRAK4 is high and they both are dual-specificity kinases. It seems likely that the last common ancestor of Pelle/IRAK kinases and plant receptor kinases already had both serine-threonine and tyrosine activity and this feature has been preserved in both animals and plants [1].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Daniel Bojar, Jacobo Martinez, Julia Santiago, Vladimir Rybin, Richard Bayliss and Michael Hothorn: Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. The Plant Journal (2014) 78, 31–43. PMID: 24461462 doi: 10.1111/tpj.12445
- ↑ Janika Witthöft, Katharina Caesar, Kirstin Elgass, Peter Huppenberger, Joachim Kilian, Frank Schleifenbaum, Claudia Oecking and Klaus Harter: The activation of the Arabidopsis P-ATPase 1 by the brassinosteroid receptor BRI1 is independent of Threonine-948 phosphorylation. Plant Signaling & Behavior 6:7, 1063-1066; July 2011. DOI: 10.4161/psb.6.7.15650
- ↑ Katharina Caesar, Kirstin Elgass, Zhonghua Chen, Peter Huppenberger, Janika Witthöft, Frank Schleifenbaum, Michael R. Blatt, Claudia Oecking and Klaus Harter: A fast brassinolide-regulated response pathway in the plasma membrane of Arabidopsis thaliana. The Plant Journal (2011) 66, 528–540. doi: 10.1111/j.1365-313X.2011.04510.x
- ↑ Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KFX & Li WH (2004) Comparative Analysis of the Receptor-like Kinase Family in Arabidopsis and Rice. The Plant Cell 16:1220-1234
- ↑ 5.0 5.1 Man-Ho Oh, Xiaofeng Wang, Steven D. Clouse and Steven C. Huber: Deactivation of the Arabidopsis Brassinosteroid Insensitive 1(BRI1) receptor kinase by autophosphorylation within the glycine-rich loop. PNAS, January 3, 2012; vol. 109, no. 1, 327–332. Doi: 10.1073/pnas.1108321109
- ↑ Man-Ho Oh, Steven D. Clouse and Steven C. Huber: Tyrosine phosphorylation in brassinosteroid signaling. Plant Signaling & Behavior 4:12, 1182-1185; December 2009. PMID: 20514242