Sandbox WWC8
From Proteopedia
Contents |
Nucleoprotein of Influenza A
|
Introduction
Viruses with anti-sense RNA genomes, such as the influenza virus carry three core polypeptides inside their viral capsids in order to successfully enter a host and initiate the viral replication cycle. Especially important for viral replication and coordination with the host cell's replication machinery is a protein able to bind single-strand RNA scripts (ssRNA), forming ribonucleoprotein complexes (RNPs). This protein is commonly referred to as nucleoprotein (NP). The NP binds and transports viral RNA scripts to and from the host cell nucleus for transcription, replication, and packaging into new virions. When NP binds RNA it is structure specific but not sequence specific, meaning NP will bind only ssRNA but will bind any ss-RNA script, viral or non-viral. Beyond the transport function, NP is an essential mediator between host and virus and coordinates complex processes during viral replication. Due to its important function, NP is intensively studied as a potential drug target for antiviral pharmaceuticals. [1] [2]
Structural Features
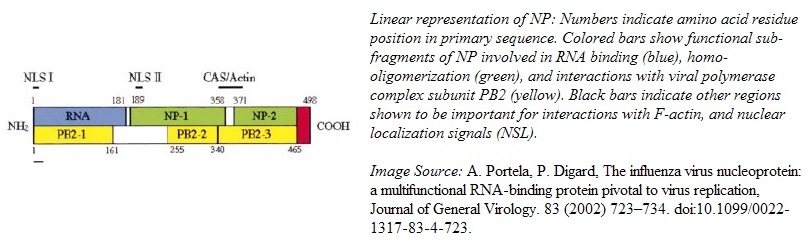
The globular NP protein is rich in arginine, serine, and glycine residues. The abundance of arginine residues gives the protein a net positive charge at pH 7. With a predicted pI of 9.3, NP is mainly composed of basic residues, except for a tail domain formed by the 30 C-terminal residues, which are acidic. The C-terminal domain has a pI of 3.7.[1] The NP is a trimer of homomers with 498 amino acid residues encoded by the Influenza A RNA segment 5. Each homomer provides a binding site for the viral RNA script.[1]
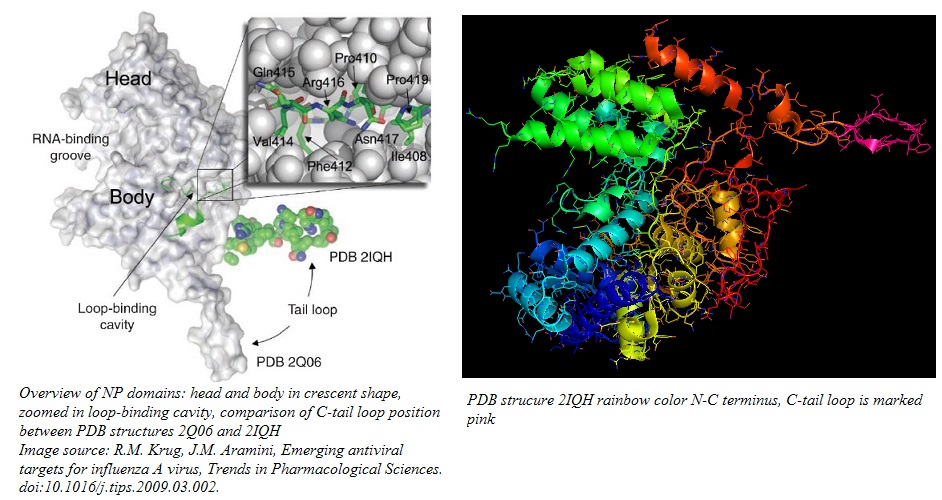
The homomers fold into a crescent shape with a head and a body domain, where the groove between the two domains hosts the ssRNA binding site on the outer surface of the homomer.[1][2] For the formation of functional RNPs, the NP needs to form a coordinated aggregate of three NPs through a process called homo-oligomerization.[2][3] The C-tail loop, marked in pink in the image below, is critical for this function. Residues 408-419, located at the back of the NP between head and tail domain, form a loop that is the basis of the interface between neighboring NPs. The C-tail loop interlocks with the loop binding cavity in the neighboring NP and forms a tight binding interaction, featuring both hydrophobic and hydrophilic residues, that keeps the trimer together. The NP oligomer conformation is especially stabilized by a salt bridge between R416 in the loop and E339 in the adjacent NP.[2]
Characterization of NP Binding Interactions and Functions in Viral Replication Cycle
RNA binding activity
NP binds ssRNA in the RNA binding groove with an affinity of ~20 nM. The binding of ssRNA is not sequence specific and the approximate stoichiometry is 1 NP monomer:24 ssRNA nucleotides. Although the portions of ssRNA that are not directly bound in the groove wrap around NP, the RNA is not protected from digestion by RNase.[1]
The RNA binding region (RNA binding groove) has been mapped (via deletion mutagenesis) to cluster around residues located in the N-terminal third of the nucleoprotein. This large region is aided in RNA binding by surrounding regions that affect the binding affinity. Thus, the RNA-binding mechanism of NP is characterized by co-operativity and allosteric regulation.[1]
Nucleoprotein Interactions with Polymerase
The influenza virus polymerase complex is essential for directing virus replication inside the host cell's nucleus. The viral polymerase complex consists of three sub units, PA, PB1, and PB2..[4] The polymerase complex directs both viral RNA replication and viral genome transcription. Additionally, specifically for viral genome transcription, the complex can steal 5' RNA primers from host mRNAs for proper transcription of viral RNA. [5] NP has been found to have substantial regulatory interaction with PB1 and PB2. The NP interaction with PB2 is host specific and depends on the amino acid sequence found in the C-terminal tail loop region. Thus, the direct interaction between NP and PB2 may be the switch between viral RNA replication (no 5' primer cap addition) and transcription (viral mRNA with added 5' primer caps).[6][2]
NP Mediator Function
NP-Importin α
Importin α is a mediator protein that interacts with hundreds of different proteins in eukaryotic cells to ensure transport across the nuclear envelope through the nuclear pores. During early stages of infection, the NP is largely concentrated in the nucleus, importing viral RNA scripts for transcription and replication. The entrance of NP into the nucleus is guaranteed by the NP interaction with Importin α.[1]
NP-CRM1
After the viral RNA scripts have been replicated, NP shuttles out of the nucleus by associating with CRM1 (chromosomal maintenance 1), also known as exportin.[7] In this later stage of infection, the concentration of NP in the cytoplasm outweighs the concentration of NP inside the nucleus. It is unknown if this is the case because the nuclear localization signals (NLS) are turned off or because the NLS are overwhelmed by the cytoplasmic accumulation signal (CAS), which describes the interaction between NP and the host cell cytoskeleton.[1]
NP-F-actin (host cell cytoskeleton)
During the late stages of the infection, the CAS region on NP acts as a cytoplasmic retention signal by tethering the nucleoprotein to the cytoskeleton. The binding between NP and F-actin was confirmed in vitro and exhibited an affinity of ~1µM with a 1:1 stoichiometry NP:F-actin..[8]
NP-M1
During packaging and assembly of new viral particles, the M1 protein (matrix protein 1 of influenza A) mediates the encapsidation of RNPs into the viral envelope. This interaction is critical for successful assembly of functional virus progeny and is characterized by direct protein-protein interaction between M1 and NP, which is stabilized by M1 simultaneously binding to the NP-bound RNA.[1]
NP Phosphorylation Profile
The influenza nucleoprotein in each strain features a fingerprint phosphorylation profile, which undergoes subtle changes during the course of a viral replication cycle. The phosphorylation profile is thought to be responsible for the diverse yet specific NP interactions at different stages in the replication cycle. Only serine residues have been found to be phosphorylated or dephosphorylated in NP. The phosphorylation profile of NP seems to be majorly influenced by the host cell's protein kinase C (PKC). In vitro inhibition of PKC by the phorbol ester 12-O-tetradecanolyphorbol 13-acetate or isoquinoline sulphonamide H7 resulted in significant phosphorylation changes in NP as well as changes in expected NP activity, proving the key role a correct NP phosphorylation profile plays in successful viral replication.[9]
Identification of Antiviral Drug Targets on NP
The large size of the RNA-binding groove on NP makes rational drug design with small molecule inhibitors difficult. Yet, NP is an attractive drug target because the protein is more conserved among influenza strains than the rapidly mutating surface proteins neuraminidase and hemagglutinin.[10] The structural exploration of the RNA-binding groove and the homo-oligomerization interface yielded promising starting points for defining new anti-viral drug targets and designing inhibitors.
Inhibition at the RNA-binding groove
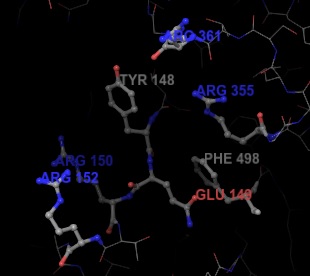
The RNA binding groove is a central focus in recent efforts to find new antiviral drugs. Inhibiting the RNA-binding activity of NP directly hinders the ability of the protein to import and export viral RNA scripts, thus slowing or stopping the replication cycle. The large size of the RNA binding groove poses a challenge to find suitable functional regions for small molecule inhibition. By the use of high-throughput techniques, S-naproxen has been identified to have sufficient binding activity on NP. The inhibitory effect of S-naproxen has been confirmed in vitro and in vivo.[10] The binding pocket around S-naproxen is defined by aromatic residuesY148 and F498, E149, and several arginine residues (R150, R152, R355, R361).[10] Current studies explore the development of better naproxen-derived inhibitors for the defined binding pocket.[11]
Inhibition by Disrupting Homo-oligomerization
The loop binding cavity may constitute a viable drug target as disruption of the homo-oligomerization process prevents the formation of functional RNPs.[2][12] Special focus lies on the disruption of the salt bridge between R416 and E339.[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 [[1]] A. Portela, P. Digard, The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication, Journal of General Virology. 83 (2002) 723–734. doi:10.1099/0022-1317-83-4-723.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 [[2]] K. Das, J.M. Aramini, L.-C. Ma, R.M. Krug, E. Arnold, Structures of influenza A proteins and insights into antiviral drug targets, Nat Struct Mol Biol. 17 (2010) 530–538. doi:10.1038/nsmb.1779.
- ↑ [[3]] Q. Ye, R.M. Krug, Y.J. Tao, The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA, Nature. 444 (2006) 1078–1082. doi:10.1038/nature05379.
- ↑ [[4]] E. Area, J. Martín-Benito, P. Gastaminza, E. Torreira, J.M. Valpuesta, J.L. Carrascosa, J. Ortín, 3D structure of the influenza virus polymerase complex: localization of subunit domains, Proceedings of the National Academy of Sciences. 101 (2004) 308–313.
- ↑ [[5]] S. Boivin, S. Cusack, R.W.H. Ruigrok, D.J. Hart, Influenza A Virus Polymerase: Structural Insights into Replication and Host Adaptation Mechanisms, J. Biol. Chem. 285 (2010) 28411–28417. doi:10.1074/jbc.R110.117531.
- ↑ [[6]] S.K. Biswas, P.L. Boutz, D.P. Nayak, Influenza Virus Nucleoprotein Interacts with Influenza Virus Polymerase Proteins, J Virol. 72 (1998) 5493–5501.
- ↑ [[7]] G. Neumann, M.R. Castrucci, Y. Kawaoka, Nuclear import and export of influenza virus nucleoprotein., J. Virol. 71 (1997) 9690–9700.
- ↑ [[8]] P. Digard, D. Elton, K. Bishop, E. Medcalf, A. Weeds, B. Pope, Modulation of Nuclear Localization of the Influenza Virus Nucleoprotein through Interaction with Actin Filaments, J Virol. 73 (1999) 2222–2231.
- ↑ [[9]] O. Kistner, K. Müller, C. Scholtissek, Differential phosphorylation of the nucleoprotein of influenza A viruses, Journal of General Virology. 70 (1989) 2421–2431.
- ↑ 10.0 10.1 10.2 [[10]] N. Lejal, B. Tarus, E. Bouguyon, S. Chenavas, N. Bertho, B. Delmas, R.W.H. Ruigrok, C.D. Primo, A. Slama-Schwok, Structure-Based Discovery of the Novel Antiviral Properties of Naproxen against the Nucleoprotein of Influenza A Virus, Antimicrob. Agents Chemother. 57 (2013) 2231–2242. doi:10.1128/AAC.02335-12.
- ↑ [[11]] B. Tarus, H. Bertrand, G. Zedda, C. Di Primo, S. Quideau, A. Slama-Schwok, Structure-based design of novel naproxen derivatives targeting monomeric nucleoprotein of Influenza A virus, Journal of Biomolecular Structure and Dynamics. 33 (2015) 1899–1912. doi:10.1080/07391102.2014.979230.
- ↑ 12.0 12.1 [[12]] A.K.-L. Ng, H. Zhang, K. Tan, Z. Li, J. Liu, P.K.-S. Chan, S.-M. Li, W.-Y. Chan, S.W.-N. Au, A. Joachimiak, T. Walz, J.-H. Wang, P.-C. Shaw, Structure of the influenza virus A H5N1 nucleoprotein: implications for RNA binding, oligomerization, and vaccine design, FASEB J. 22 (2008) 3638–3647. doi:10.1096/fj.08-112110.