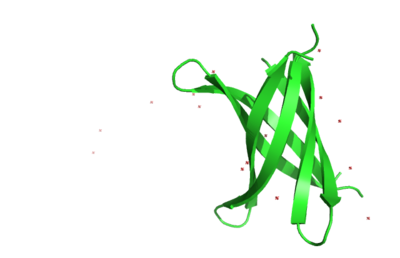
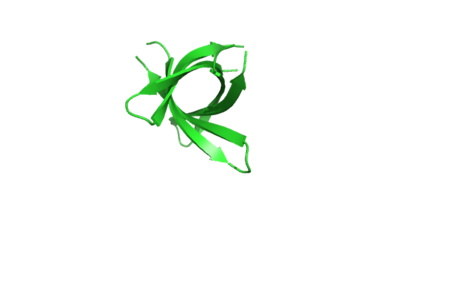
The interactive Molecular Tour below assumes that you are familiar with the journal article[1].
αβ
Introduction
Amyloid fibrils were first assumed to be the agents of Amyloid diseases,including Alzheimer’s,Parkinson’s and the prion conditions.But studies from many laboratories suggest that the reason for this disorder are lower molecular weight entities known as small amyloid oligomers,instead of the associated protein fibrils.Segment of amyloid forming protein makes oligomeric complex which exhibits properties of other amyloid oligomers.They are rich in beta-sheet structure and these oligomer can be identified by a conformational antibody(A11) that binds oligomers but not fibrils,irrespective of sequence of constituent protein.This protein is a chaperone that forms amyloid fibrils.The structure of oligomer shows a cylindrical barrel,made up of six anti-parallel protein strands known as cylindrin.This segment(coloured in black) termed as forms the cylindrin structure.
Molecular Tour
Oligomer forming segment of ABC(αβ crystallin) were identified by inspection of its 3D structure and by applying the Rosetta-Profile algorithm to its sequence(Fig 1).Two segments of high amyloidogenic propensity,with sequences and (where D indicates Asp; E, Glu; G, Gly; I, Ile; K, Lys; and V, Val).
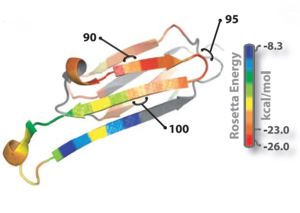
Fig. 1 : Ribbon diagram of a single subunit of ABC (16), colored by propensity to form amyloid, with red being the highest and blue the lowest propensity. The segment from residue 90 to 100, termed K11V, forms the cylindrin.
The entire 11-residue segment KVKVLGDVIEV forms a in the 3D structure of ABC.The six residue segment GDVIEV ,termed ,forms fibrils and microcrystals.The microcrystals enabled us to determine the atomic structure of G6V, which proved to be a standard class 2 steric zipper, essentially an amyloid-like protofilament.
Amyloid fibrils and oligomer are both formed by the KVKVLGDVIEV.K11V forms fibrils similar to those of protein(ABC) on shaking at elevated temperature and also similar to K11VV2L ().The fibrils diameter range from 20 to 100 nm in electrom microscope.G6V,K11V,K11V-TR are all convertible to amyloid state,as is their parent protein ABC.Segment K11V,K11V-TR,and a sequence variant with Leu replacing Val at position 2( K11VV2L ) are capable of converting to amyloid state as their parent protein ABC and forms stable oligomers intermediate in size between monomer and fiber.
ABC K11V oligomers exhibit molecular properties in common with amyloid oligomers from other disease-related proteins and oligomers were observed to be toxic ,displaying dose-response effects similar to those of alpha-beta involved in Alzheimer's disease.
K11V and K11VV2L form hexameric oligomers.K11V oligomer is of 6 chains and K11V-TR oligomer of three tandem chains.Other than the glycine linkers and the Val-to-Leu replacement, the cylindrical bodies of the six stranded K11V and the three double stranded K11V-TR oligomers are essentially identical.
The structure of K11V is a six-stranded antiparallel barrel of cylindrical in shape also called as cylindrin(Fig. 3 & Fig.4).It is completely different in structure from either the native structure of ABC or from G6V segment.Each strand of cylindrin is bonded to one neighbouring strand by a strong interface and to a second by a weak interface.The weak interface is formed by eight hydrogen bonds: four from the main chain, two mediated through side-chain interactions, and two through a water bridge.The strong interface is formed by 12 hydrogen bonds and spreads outward at the ends.

Fig. 3 Ribbon representation of the cylindrin crystal structure.
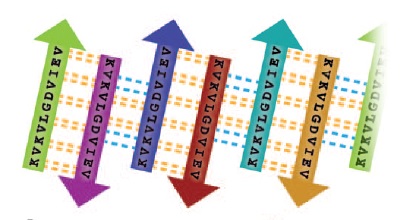
Fig. 4 Schematic of unrolled cylindrin (outside view), illustrating strand-to-strand registration. Hydrogen bonds between the main chains of neighboring strands are shown by yellow dashed lines; hydrogen bonds mediated by water bridges or side chains are shown by blue dashed lines.
The structure of K11V-TR was determined, even though glycine linkers and Val-to-Leu replacement,cylindrical bodies of three double stranded K11V-TR oligomers and six-stranded K11V were identical.
There are proofs that amyloid oligomers are beta-sheet rich,and many toxic oligomers are recognized by A11 conformational antibody,which also recognizes cylindrin.Threfore, the cylindrin structure may represent amyloid oligomer's common structural core.
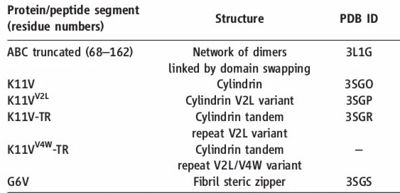
Fig. 7 Information about amyloid related oligomers,derived from ABC
PDB's of the oligomers
PDB'S are
K11V (), K11V-Br2 (), K11V-Br8 (), K11VV2L (), K11V-TR (), and GDVIEV ().
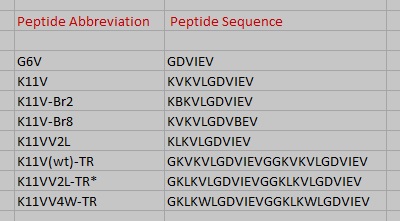
Fig. 8 Cylindrin single chain and tandem repeat peptide abbreviations and amino acid sequences.