Sandboxjg
From Proteopedia
ClC Transporter
|
is a membrane protein Escherichia coli which belongs to the CLC family of ion channels and transporters. These proteins are essential for the maintenance of proper membrane potential in muscle cells, for the transport of electrolytes across epithelial layers, and to. Roderick MacKinnon and his team determined the structure of this protein and proposed that it was a Cl- selective ion channel. Accardi and Miller showed that CLC-ec1 functions as a transporter: it exchanges 2 Cl- :1 H+. Image:ClCvideoProteopedia.mov.
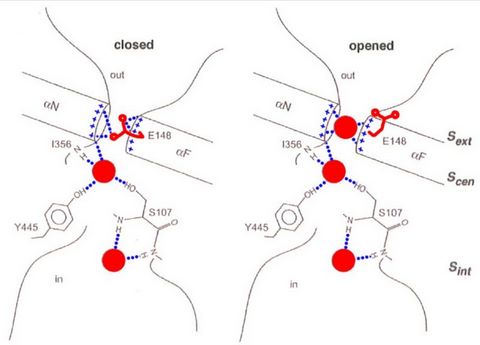
The CLC-ec1 transporter is a dimer formed of two polypeptide chains each containing an internal repeat arranged in an anti-parallel organization. Each monomer functions independently of the other and creates a passage for ions through the membrane The Cl- and H+ pathways are formed by an extensive network of interactions between the protein and substrates. The Cl- ions are stabilized in the middle of the membrane by the dipole moment of two a-helices, by interactions with amides from the protein’s backbone and by the direct coordination of two conserved side chains. Because each polypeptide chain functions independently, we will focus on the structure of one pore.
There are 3 chloride binding sites in each monomer, an exterior binding site, a central binding site, and an interior binding site. These three sites span the membrane and define the transport pathway for Cl-.One of the interesting properties of this protein family is that its members can function either as ion channels or transporters. Furthermore, mutating Glu 148 to Ala in CLC-ec1 eliminates H+ transport but chloride ions can still move freely through the protein giving rise to a channel-like behavior. Mutations in other family members cause myotonia congenital (CLC-1), osteopetrosis (CLC-7) and kidney pathologies such as Bartter’s syndrome (CLC-Ka and -Kb) and Dent’s disease (CLC-5).
To see the test