Structure and dynamics of the microtubule-binding hot-spots on the neuronal protein tau
From Proteopedia
Contents |
Abstract
Intrinsically disordered protein tau plays a prominent role in the regulation of microtubular dynamics and axonal development. It binds to microtubules, supports their polymerization and organizes their assembly into evenly spaced bundles. The sites on tau molecule involved in the interaction with microtubules have been described with using of several methods (e.g. truncation analysis [1], NMR spectrometry [2], phage display [3]). Tau protein undergoes many posttranslational modifications as phosphorylation [4] and truncation [5], which can play a role in the onset of neurodegeneration. Under disease conditions (e.g. Alzheimer’s disease) tau dissociates from microtubules, misfolds and creates highly insoluble paired helical filaments (PHFs).
For the presented study, two monoclonal antibodies (DC25, Axon Neuroscience SE, Bratislava, Slovakia; and Tau5 [6]) with the epitopes in the hotspots of tau microtubule binding have been chosen. The affinity and binding enthalpy and entropy of their interaction with full length and truncated tau variants have been evaluated from the kinetic measurements obtained with the surface plasmon resonance. To elucidate the structure of tau in the hotspots of microtubule interaction, the Fab fragments of used monoclonal antibodies were crystallized alone and with 30 amino-acid long tau peptides. From two pairs of crystalized free Fab fragment and Fab complexed with tau peptide, three complete datasets were measured.
Determination of kinetic and thermodynamic characteristics of antibody-tau interaction and their changes after tau truncation
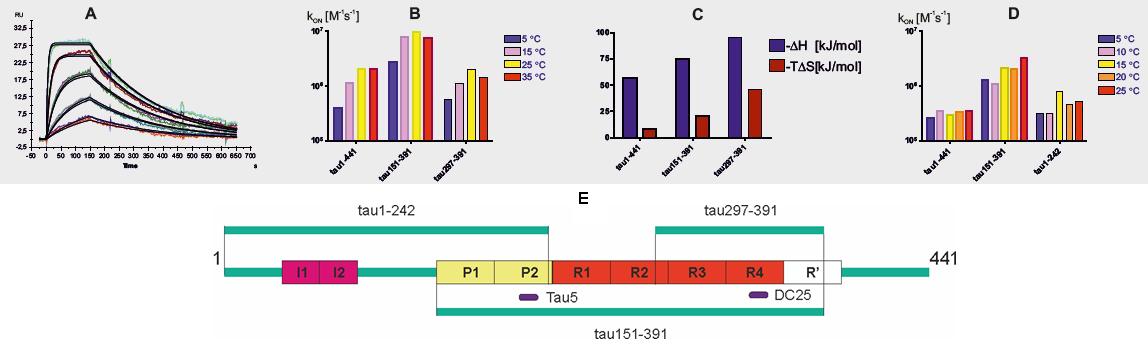
The complex formation between the full length and truncated tau proteins and Fab fragments of DC25 and Tau5 monoclonal antibodies has been monitored by the surface plasmon resonace. (A) The binding curves for interaction between full length tau and DC25 Fab fragment at 25°C. (B) The comparison of the association rate constants kON obtained from SPR measurements at various temperatures shows that truncated tau151-391 differs substantially in association kinetics.(C) The equilibrium dissociation constants obtained at various temperature points allow us to calculate the binding enthalpy and entropy from the Van’t Hoff equation [7]. The results calculated for the interaction of tau proteins with DC25 Fab fragment show that the truncated tau proteins exhibit higher negative binding enthalpy and entropy than full length tau. As the contact sites in complex are the same for all tau proteins, changes in enthalpy reflect different internal bonds in full length and truncated tau proteins. Truncated tau proteins also exhibit larger negative entropy upon the complex formation so the relative fixation of truncated tau proteins in complex is higher than that of full length tau, which has implications for the interactiontruncated tau with its cellular partners. (D) The comparison of the association rate constants kON for binding of tau proteins to Tau5 Fab fragment exhibits similar dependency on truncation as in the case of DC25. (E) The schema of tau varians and antibody epitopes used in this work. The binding of full length tau, tau151-391 (4 repeat) and tau297-391 [8] to the DC25 Fab fragment was investigated. For Tau5 Fab, the tau1-242 was used instead of tau297-391 as a truncated tau variant serving as a control to truncated tau151-391, which is sufficient to drive a pathology of Alzheimer's type in a rat model [9], [10].
Preliminary structure solution of DC25 Fab fragment
The structure of DC25 Fab fragment is being solved by the molecular replacement method using the Phaser program [11]. Initially we have used the Fab fragment of monoclonal antibody MN423 , which binds to the C-terminus of truncated tau [12] and has been also crystallized (by our group) in complex with the terminal hexapeptide
|
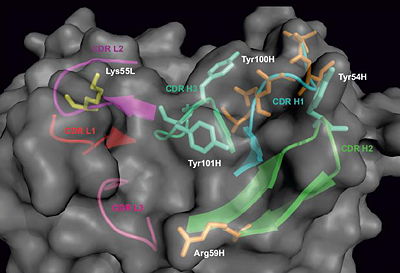
The residues which have undergone somatic hypermutation in the process of antibody affinity maturation were identified by sequence comparison with the antibody germline and are highlighted in orange. Among them, Arg59H could mediate the contact with antigen. The tyrosine residues in heavy chain CDRs H2 and H3 can also mediate the contacts with antigen. Lys55L in the CDR L2 can be also involved in antigen binding, as it is in a complex structure involving nearly identical light chain (PDB code 2hkf [17]). Proposed constraints for antigen binding will be used for molecular docking of the epitope sequence (KDRVQSK) into the obtained paratope structure of DC25 antibody.
Summary
Three complete datasets have been collected, for DC25 and Tau5 Fab fragments and for the complex of Tau5 fab fragment with peptide tau201-230. The structures will be solved by molecular replacement and will deepen the understanding of tau physiology. The data obtained with surface plasmon resonance have revealed the significant difference between full length and truncated tau mainly in association kinetics.
References
- ↑ Goode BL, Chau M, Denis PE, Feinstein SC. Structural and functional differences between 3-repeat and 4-repeat tau isoforms. Implications for normal tau function and the onset of neurodegenetative disease. J Biol Chem. 2000 Dec 8;275(49):38182-9. PMID:10984497 doi:10.1074/jbc.M007489200
- ↑ Mukrasch MD, Bibow S, Korukottu J, Jeganathan S, Biernat J, Griesinger C, Mandelkow E, Zweckstetter M. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009 Feb 17;7(2):e34. PMID:19226187 doi:10.1371/journal.pbio.1000034
- ↑ Cao B, Mao C. Identification of microtubule-binding domains on microtubule-associated proteins by major coat phage display technique. Biomacromolecules. 2009 Mar 9;10(3):555-64. PMID:19186939 doi:10.1021/bm801224q
- ↑ Alonso AD, Di Clerico J, Li B, Corbo CP, Alaniz ME, Grundke-Iqbal I, Iqbal K. Phosphorylation of tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J Biol Chem. 2010 Oct 1;285(40):30851-60. Epub 2010 Jul 27. PMID:20663882 doi:10.1074/jbc.M110.110957
- ↑ Kovacech B, Novak M. Tau truncation is a productive posttranslational modification of neurofibrillary degeneration in Alzheimer's disease. Curr Alzheimer Res. 2010 Dec;7(8):708-16. PMID:20678071
- ↑ LoPresti P, Szuchet S, Papasozomenos SC, Zinkowski RP, Binder LI. Functional implications for the microtubule-associated protein tau: localization in oligodendrocytes. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10369-73. PMID:7479786
- ↑ Tellinghuisen J. Van't Hoff analysis of K degrees (T): how good...or bad? Biophys Chem. 2006 Mar 20;120(2):114-20. Epub 2005 Nov 21. PMID:16303233 doi:10.1016/j.bpc.2005.10.012
- ↑ Novak M, Kabat J, Wischik CM. Molecular characterization of the minimal protease resistant tau unit of the Alzheimer's disease paired helical filament. EMBO J. 1993 Jan;12(1):365-70. PMID:7679073
- ↑ Zilka N, Filipcik P, Koson P, Fialova L, Skrabana R, Zilkova M, Rolkova G, Kontsekova E, Novak M. Truncated tau from sporadic Alzheimer's disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett. 2006 Jun 26;580(15):3582-8. Epub 2006 May 22. PMID:16753151 doi:10.1016/j.febslet.2006.05.029
- ↑ Filipcik P, Zilka N, Bugos O, Kucerak J, Koson P, Novak P, Novak M. First transgenic rat model developing progressive cortical neurofibrillary tangles. Neurobiol Aging. 2012 Jul;33(7):1448-56. Epub 2010 Dec 31. PMID:21196063 doi:10.1016/j.neurobiolaging.2010.10.015
- ↑ McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007 Aug 1;40(Pt 4):658-674. Epub 2007 Jul 13. PMID:19461840 doi:10.1107/S0021889807021206
- ↑ Khuebachova M, Verzillo V, Skrabana R, Ovecka M, Vaccaro P, Panni S, Bradbury A, Novak M. Mapping the C terminal epitope of the Alzheimer's disease specific antibody MN423. J Immunol Methods. 2002 Apr 1;262(1-2):205-15. PMID:11983234
- ↑ Skrabana R, Dvorsky R, Sevcik J, Novak M. Monoclonal antibody MN423 as a stable mold facilitates structure determination of disordered tau protein. J Struct Biol. 2010 Feb 23. PMID:20184958 doi:10.1016/j.jsb.2010.02.016
- ↑ Mareeva T, Martinez-Hackert E, Sykulev Y. How a T cell receptor-like antibody recognizes major histocompatibility complex-bound peptide. J Biol Chem. 2008 Oct 24;283(43):29053-9. Epub 2008 Aug 14. PMID:18703505 doi:10.1074/jbc.M804996200
- ↑ Winn MD, Murshudov GN, Papiz MZ. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 2003;374:300-21. PMID:14696379 doi:10.1016/S0076-6879(03)74014-2
- ↑ Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004 Dec;60(Pt 12 Pt 1):2126-32. Epub 2004, Nov 26. PMID:15572765 doi:10.1107/S0907444904019158
- ↑ Kral V, Mader P, Collard R, Fabry M, Horejsi M, Rezacova P, Kozisek M, Zavada J, Sedlacek J, Rulisek L, Brynda J. Stabilization of antibody structure upon association to a human carbonic anhydrase IX epitope studied by X-ray crystallography, microcalorimetry, and molecular dynamics simulations. Proteins. 2008 May 15;71(3):1275-87. PMID:18041760 doi:10.1002/prot.21821