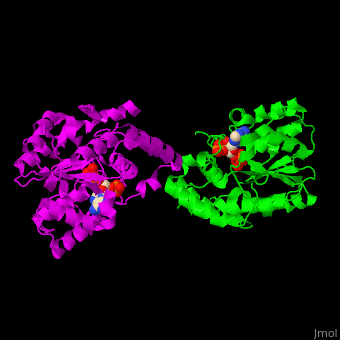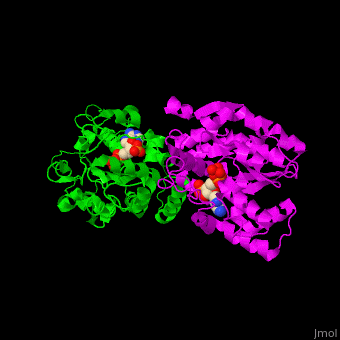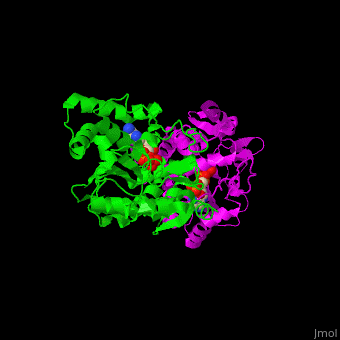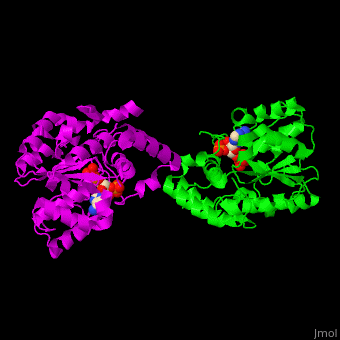
Sulfotransferase
From Proteopedia
| |||||||||||
References
- ↑ Malojcic G, Glockshuber R. The PAPS-independent aryl sulfotransferase and the alternative disulfide bond formation system in pathogenic bacteria. Antioxid Redox Signal. 2010 Oct;13(8):1247-59. doi: 10.1089/ars.2010.3119. PMID:20136513 doi:http://dx.doi.org/10.1089/ars.2010.3119
- ↑ Yi M, Negishi M, Lee SJ. Estrogen Sulfotransferase (SULT1E1): Its Molecular Regulation, Polymorphisms, and Clinical Perspectives. J Pers Med. 2021 Mar 11;11(3):194. PMID:33799763 doi:10.3390/jpm11030194
- ↑ Teixeira FCOB, Vijaya Kumar A, Kumar Katakam S, Cocola C, Pelucchi P, Graf M, Kiesel L, Reinbold R, Pavão MSG, Greve B, Götte M. The Heparan Sulfate Sulfotransferases HS2ST1 and HS3ST2 Are Novel Regulators of Breast Cancer Stem-Cell Properties. Front Cell Dev Biol. 2020 Sep 25;8:559554. PMID:33102470 doi:10.3389/fcell.2020.559554
- ↑ Ouyang Yb, Lane WS, Moore KL. Tyrosylprotein sulfotransferase: purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2896-901. PMID:9501187 doi:10.1073/pnas.95.6.2896
- ↑ Nagar S, Walther S, Blanchard RL. Sulfotransferase (SULT) 1A1 polymorphic variants *1, *2, and *3 are associated with altered enzymatic activity, cellular phenotype, and protein degradation. Mol Pharmacol. 2006 Jun;69(6):2084-92. PMID:16517757 doi:10.1124/mol.105.019240
- ↑ Engelke CE, Meinl W, Boeing H, Glatt H. Association between functional genetic polymorphisms of human sulfotransferases 1A1 and 1A2. Pharmacogenetics. 2000 Mar;10(2):163-9. PMID:10762004 doi:10.1097/00008571-200003000-00008
- ↑ Bian HS, Ngo SY, Tan W, Wong CH, Boelsterli UA, Tan TM. Induction of human sulfotransferase 1A3 (SULT1A3) by glucocorticoids. Life Sci. 2007 Dec 14;81(25-26):1659-67. PMID:17963788 doi:10.1016/j.lfs.2007.09.029
- ↑ Dubaisi S, Fang H, Kocarek TA, Runge-Morris M. Transcriptional Regulation of Human Cytosolic Sulfotransferase 1C3 by Peroxisome Proliferator-Activated Receptor γ in LS180 Human Colorectal Adenocarcinoma Cells. Mol Pharmacol. 2016 Nov;90(5):562-569. PMID:27565680 doi:10.1124/mol.116.106005
- ↑ Schrag ML, Cui D, Rushmore TH, Shou M, Ma B, Rodrigues AD. Sulfotransferase 1E1 is a low km isoform mediating the 3-O-sulfation of ethinyl estradiol. Drug Metab Dispos. 2004 Nov;32(11):1299-303. PMID:15483196 doi:10.1124/dmd.32.11
- ↑ Wong T, Wang Z, Chapron BD, Suzuki M, Claw KG, Gao C, Foti RS, Prasad B, Chapron A, Calamia J, Chaudhry A, Schuetz EG, Horst RL, Mao Q, de Boer IH, Thornton TA, Thummel KE. Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D(3)-3-O-Sulfate, a Major Circulating Vitamin D Metabolite in Humans. Drug Metab Dispos. 2018 Apr;46(4):367-379. PMID:29343609 doi:10.1124/dmd.117.078428
- ↑ Falany CN, He D, Dumas N, Frost AR, Falany JL. Human cytosolic sulfotransferase 2B1: isoform expression, tissue specificity and subcellular localization. J Steroid Biochem Mol Biol. 2006 Dec;102(1-5):214-21. PMID:17055258 doi:10.1016/j.jsbmb.2006.09.011
- ↑ Minchin RF, Lewis A, Mitchell D, Kadlubar FF, McManus ME. Sulfotransferase 4A1. Int J Biochem Cell Biol. 2008;40(12):2686-91. PMID:18248844 doi:10.1016/j.biocel.2007.11.010
- ↑ Teramoto T, Nishio T, Kurogi K, Sakakibara Y, Kakuta Y. The crystal structure of mouse SULT2A8 reveals the mechanism of 7alpha-hydroxyl, bile acid sulfation. Biochem Biophys Res Commun. 2021 May 21;562:15-20. doi:, 10.1016/j.bbrc.2021.04.113. PMID:34030040 doi:http://dx.doi.org/10.1016/j.bbrc.2021.04.113
- ↑ Paul P, Suwan J, Liu J, Dordick JS, Linhardt RJ. Recent advances in sulfotransferase enzyme activity assays. Anal Bioanal Chem. 2012 Jun;403(6):1491-500. PMID:22526635 doi:10.1007/s00216-012-5944-4
- ↑ Yalcin EB, More V, Neira KL, Lu ZJ, Cherrington NJ, Slitt AL, King RS. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab Dispos. 2013 Sep;41(9):1642-50. doi: 10.1124/dmd.113.050930. Epub 2013, Jun 17. PMID:23775849 doi:http://dx.doi.org/10.1124/dmd.113.050930
- ↑ Pedersen LC, Petrotchenko E, Shevtsov S, Negishi M. Crystal structure of the human estrogen sulfotransferase-PAPS complex: evidence for catalytic role of Ser137 in the sulfuryl transfer reaction. J Biol Chem. 2002 May 17;277(20):17928-32. Epub 2002 Mar 7. PMID:11884392 doi:http://dx.doi.org/10.1074/jbc.M111651200