Template:HMGR Medical
From Proteopedia
Medical Implications
Elevated cholesterol levels have been identified as a major risk factor for coronary artery disease, the narrowing of arteries of the heart, which affected over 13 million people in the United States alone. It is a major cause of disability and death, killing over 500 thousand people in the USA in 2001. [1]
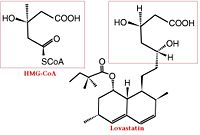
The statins are HMG-CoA reductase inhibitors. Discovered by Akira Endo in 1971, statins are similar in structure to HMG-CoA and act by competitively inhibiting HMGR. Since HMGR is the first committed enzyme in the cascade that eventually produces cholesterol, use of statins can dramatically reduce blood cholesterol levels.[2] As a drug class, statins generated over $20 billion dollars in sales in 2009 with Pfizer’s Lipitor being the best selling drug in the world at the time of writing.[3][4]
A number of crystal structures of HMGR with bound statins have been solved which elucidate how the statin molecule is bound by HMGR. Statins in general occupy the active site of HMGR, preventing HMG-CoA from binding. The structure of HMGR with bound statins () shows that the cis loop forms a number of polar interactions with the statin inhibitor, particularly residues Ser 684, Asp 690, Lys 691, Lys 692, and hydrogen bond interactions between Glu 559 and Asp 767 with the O5-hydroxyl of the statins. Van der Waals interactions between Leu 562, Val 683, Leu 853, Ala 856, and Leu 857 of HMGR and hydrophobic ring structures of the statins contribute to binding as well.[5] These interactions result in the statins binding to HMGR with a Ki of between .1-2.3nM while the Michaelis constant KM for HMG-CoA is 4uM, allowing the statins to outcompete HMG-CoA in binding to HMGR.[6] Additional structures of HMGR with the statins , , , , and highlight the important residues involved in inhibitor binding.