Introduction
Urease is a nickel-dependent metalloenzyme, is synthesized by plants, some bacteria, and fungi [1].
Jack bean urease was the first enzyme to be crystallized, accomplished by James. B. Sumner in 1926, one for which he was awarded Nobel Prize in chemistry in 1946 [2]. Like urease, its substrate urea is also of major historical significance since it was the first organic compound to be synthesized in 1828. Urea is a major nitrogenous waste product of biological actions. In general, urea is short-lived and rapidly metabolized by microbial activities. Urease catalyzes the hydrolysis of urea to form ammonia and carbamate. The compound spontaneously hydrolyzes at physiological pH to form carbonic acid and a second molecule of ammonia [3].
Ureases are among the few enzymes that require nickel for activity. It is known that binding of nickel to urease is very specific and tight and the removal of metal ions can be achieved only by harsh treatment with denaturants or acids,[4] which is not the case in most other metalloenzymes. In vivo incorporation of nickel in both bacterial and plant ureases requires a set of accessory proteins that appear to act as urease-specific chaperones [5].
One of the most common bacterial urease is the Helicobacter pylori since it has been implicated in peptic ulcers and stomach cancer [6]. In plants, urease is widely distributed in leguminous seeds and is suggested to play an important role in seed germination[6]. Plant ureases are also suggested to participate in seed chemical defenses [7].
See also Urease (Hebrew).
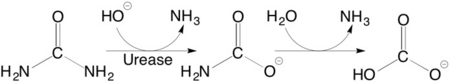
Reaction
The reaction occurs as follows:
(NH2)2CO + H2O → CO2 + 2NH3
Characteristics[8]
Urease (Urea Amidohydrolase EC 3.5.1.5) catalyzes the hydrolysis of urea to ammonia and carbon dioxide, thus allowing organisms to use exogenous and internally generated urea as a nitrogen source[1].
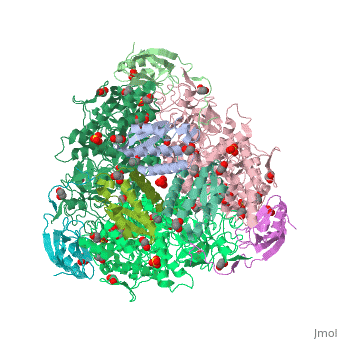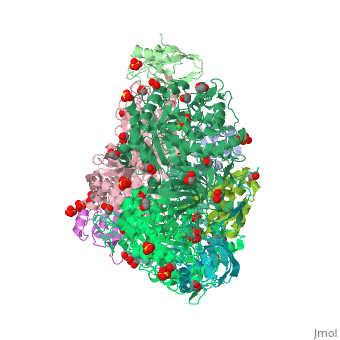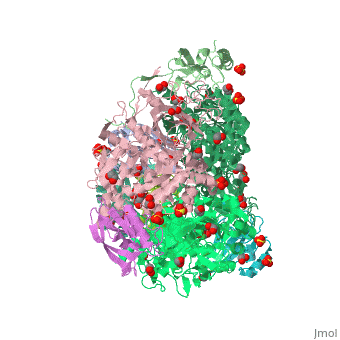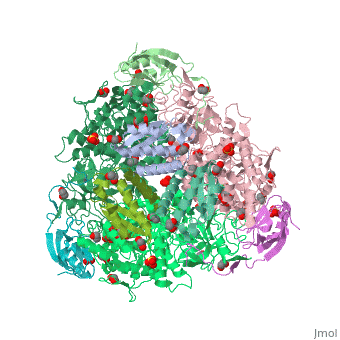
The multi-subunit enzyme usually has a 3:3 (alpha:beta) stoichiometry with a 2-fold symmetric structure (note that the image above gives the structure of the asymmetric unit, one-third of the true biological assembly). An exceptional urease is found in Helicobacter pylori, which combines four of the regular six-subunit enzymes in an overall tetrahedral assembly of 24 subunits (α12β12). This supra-molecular assembly is thought to confer additional stability for the enzyme in this organism, which functions to produce ammonia in order to neutralise gastric acid. The presence of urease is used in tUpdate February 2013he diagnosis of Helicobacter species[8].
Molecular weight: 480 kDa or 545 kDa for Jack Bean Urease
Optimum pH: 7.4
Optimum Temperature: 60 degrees Celsius
Enzymatic Specificity: Urea and Hydroxyurea
Inhibitors: Heavy Metals (Pb- & Pb2+)
Structural Properties
Plant ureases are made up of single-chain polypeptide in contrast to bacterial ureases, which consist of two or three polypeptides designated as alpha, beta and gamma [9].
In the native enzyme, the coordination sphere of each of the two nickel ions is completed by a water molecule and a bridging hydroxide. A fourth water molecule completes a tetrahedral cluster of solvent molecules. The enzyme crystallized in the presence of phenylphosphorodiamidate contains the tetrahedral transition-state analogue diamidophosphoric acid, bound to the two nickel ions in an unprecedented mode. Comparison of the native and inhibited structures reveals two distinct conformations of the flap lining the active-site cavity [10].
The major structural difference observed between plant urease and bacterial ureases are at the gap regions between the alpha, beta and gamma subunits and at a loop region that covers the active site[1]. Interestingly, the structural analysis of the plant urease JBU (Jack Bean Urease) revealed the presence of extensive intermolecular interactions in the hexameric assembly, which would provide the structure-based explanation for the enzymes remarkable stability[1].
The active site of plant urease is similar to that of the bacterial urease, consisting of bi-nickel centre with nickel ions Ni1 and Ni2, separated by a distance of 3.7 Å. Residues His519, His545, and Lys 490 are liganded to Ni1, while the residues His407, His409, Asp633 and Lys490 are liganded to Ni2. Lys490 is carbamylated and acts as a bridging residue between the two nickels [1]. In bacterial ureases, a mobile flap that covers the active site was suggested to play an important structural feature by exhibiting two different conformations through which the enzyme regulates both access of the substrate to the active site and the release of the reaction products[1].
Function
Many gastrointestinal or urinary tract pathogens produce urease, enabling the detection of urease to be used as a diagnostic tool to detect presence of pathogens [11].
Plant and fungal ureases are homo-oligomeric proteins of 90-kDa identical subunits, while bacterial ureases are multimers of two- or three-subunit complexes. The bacterial and plant ureases have high sequence similarity, suggesting that they have similar three-dimensional structures and a conserved catalytic mechanism [9][12].
Both bacterial and plant ureases display several biological activities that are independent of their ureolytic activity[13]. For example, enzymatic activity is not involved in platelet aggregation and antifungal activities of plant and microbial ureases[14].
Similarly, the lethal activity of canatoxin in mice and the insecticidal activity of plant ureases are independent of ureolytic activity[14]. It is interesting to note that, in spite of their closely related amino acid sequences, the insecticidal activity of ureases differ significantly among plant and bacterial ureases.
H. pylori expresses a large amount of urease and levels can reach up to 10% of total cellular protein. Urease contains 12 nickel atoms per molecule, and thus H. pylori has a relatively high demand for nickel. Urease plays a central role in the pathogenesis of H. pylori infection and catalyzes the conversion of urea into carbon dioxide and ammonia. The latter is able to neutralize gastric acid and offer protection to H. pylori against the low pH in the stomach[9].
Mechanism of Urease Activity
Two mechanisms have been proposed for crystal structures of native, site-directed variants, and inhibitor complexes of bacterial ureases K. Aerogenes and B. pasteurli [1].
In the enzymatic mechanism based on the crystal structure of K. aerogenes enzyme, urea binds with its carbonyl oxygen bound to Ni1 and retaining a water molecule in the Ni2 site. Consequently, the active-site flap closes and the Ni2-bound hydroxide acts as a nucleophile and attacks the carbonyl carbon atom of the urea molecule, which is polarized by coor- dination to Ni1. The reaction proceeds through a tetrahedral intermediate that releases ammonia with His320 acting as a general acid [15]. In the other mechanism for B. pasteurii enzyme, urea binds in a bidentate manner with its carbonyl oxygen bound to Ni1 and one of the amino group bound to Ni2, thus replacing three water moieties, leaving only the bridging hydroxide. This hydroxide attacks urea to give the tetrahedral transition state leading to formation of ammonia and carbamate [16].
Medical Significance and Future Implications
People with genetic defects in any enzyme involved in urea formation cannot tolerate protein-rich diets[17]. Amino acids ingested in excess of the minimum daily requirements for protein synthesis are deaminated in the liver, producing free ammonia that cannot be converted to urea and exported into the bloodstream, as ammonia is highly toxic. The absence of urea cycle enzyme can result in hyperammonemia[18] or in the build up of one or more urea cycle intermediates, depending on the enzyme that is missing. Given that most urea cycle steps are irreversible, the absent enzyme activity can often be identified by determining which cycle intermediate is present in especially elevated concentration in the blood and/or urine. Although the breakdown of amino acids can have serious health consequences in individuals with urea cycle deficiencies, a protein-free diet is not a treatment option. Humans are incapable of synthesizing half of the 20 amino acids, and these essential amino acids must be provided in diet[17].
A variety of treatments are available for individuals with urea cycle defects. Careful administration of the aromatic acids benzoate or phenyl butyrate in the diet can help lower the level of ammonia in the blood[19].
The crystal structure of Sporosarcina pasteurii urease in a complex with citrate provides new hints for inhibitor design [20]
, the enzyme that catalyses the hydrolysis of urea, is a virulence factor for a large number of ureolytic bacterial human pathogens. The increasing resistance of these pathogens to common antibiotics, as well as the need to control urease activity to improve the yield of soil nitrogen fertilisation in agricultural applications, has stimulated the development of novel classes of molecules that target urease as enzyme inhibitors. We report on the crystal structure of a from Sporosarcina pasteurii, a widespread and highly ureolytic soil bacterium, with 1.50 Å resolution. The fit of the ligand to the involves stabilising interactions, such as a carboxylate group that binds the nickel ions at the active site and several hydrogen bonds with the surrounding residues. The nitrogen, oxygen and nickel atoms are blue, red, and green, respectively. The carbon atoms of citrate are in yellow. The compared with previously reported ligands co-crystallised with urease and thus represents a unique and promising scaffold for the design of new, highly active, stable, selective inhibitors. The residues which interact with Ni and OH are in darkmagenta, of note, His249, His139, and Kcx220[21], whereas the residues which interact with citrate are in magenta.
Selectivity of Ni(II) and Zn(II) binding to Sporosarcina pasteurii UreE, a metallo-chaperone in the urease assembly: a calorimetric and crystallographic study [22]
Urease is a nickel-dependent enzyme that plays a critical role in the biogeochemical nitrogen cycle by catalyzing the hydrolysis of urea to ammonia and carbamate. This enzyme, initially synthesized in the apo-form, needs to be activated by nickel ion incorporation into the active site, driven by the dimeric metallo-chaperone UreE. The present study explores the metal selectivity and affinity of UreE from Sporosarcina pasteurii for cognate (Ni(II)) and non-cognate (Zn(II)) metal ions. The , polypeptide chain A and B are shown in green and darkmagenta respectively, Ni ion shown as a cyan ball, Zn ion shown as a grey ball, two His100 shown in ball-and-stick representation and colored in magenta, nitrogen atoms are in blue and oxygen atoms are in red. The protein chains do not form a dimer of dimers in the
crystal lattice, but arranged around the 63 axis, forming a large solvent channel. . The , linking symmetry-related dimers (colored in salmon), and coordinated with a pseudo-tetrahedral geometry, interacting with from a symmetry-related dimer. In particular, the thermodynamic parameters of SpUreE for Ni(II) and Zn(II) binding have been determined using isothermal titration calorimetry. These experiments show that two Ni(II) ions bind to the protein dimer with positive cooperativity, with a high affinity and a low affinity site. Zn(II) binding to the protein, occurring in the same region and with similar affinity, causes metal-driven dimerization of the protein dimer. The crystal structure of the protein obtained in the presence of equimolar amounts of both metal ions indicates that the high affinity metal binding site preferentially binds Ni(II) over Zn(II). The ability of the protein to select Ni(II) over Zn(II) was confirmed by competition experiments in solution as well as by analysis of X-ray anomalous dispersion data. Overall, the thermodynamics and structural parameters that modulate the metal ion specificity of different binding sites on the protein surface have been established.
Evidence-based docking of the urease activation complex
Evidence-based docking of the [23]
Ureases are enzymes that break down urea to carbon dioxide and ammonia, and they are one of the very few enzymes that have nickel in their active sites. Genetic and biochemical studies have shown that most of these enzymes require accessory proteins for the correct assembly of the nickel in their metallocenters. (green), (red), and (darkmagenta) form the . The trimeric representation
considers UreABC as a functional unit. Studies of Klebsiella aerogenes urease activation pathway revealed that three accessory proteins – UreD (yellow), UreF (cyan), UreG (magenta) – are essential for the production of a functional urease. These proteins sequentially bind to form the , , and activation complexes. is rotated by 90º. In this work we submitted structural models of such proteins to macromolecular docking calculations with K. aerogenes urease, which lead to a putative structure for the urease activation complex.
The presented model for this complex is the first to include UreG and to use the current data on the activation pathway to guide the docking calculations. Despite the urease activation process being far more complex, our results are likely to expand the current knowledge on this essential step for proper ureolytic activity, aiding further high resolution studies of this macromolecular assembly by providing a 3D scaffold to work upon.
Fluoride inhibition of Sporosarcina pasteurii urease: structure and thermodynamics [24]
(it is in homotrimeric form of αβγ heterotrimer) is a nickel-dependent enzyme (Ni(II) ions are shown as green spheres, α, β, and γ subunits are colored in darkmagenta, yellow, deeppink, respectively) and a virulence factor for ureolytic bacterial human pathogens, but it is also necessary to convert urea (see static image below), the most worldwide used fertiliser, into forms of nitrogen that can be taken up by crop plants.
A strategy to control the activity of urease for medical and agricultural applications is to use enzyme inhibitors. Fluoride is a known urease inhibitor, but the structural basis of its mode of inhibition are still undetermined. Here, kinetic studies on the fluoride-induced inhibition of urease from Sporosarcina pasteurii, a widespread and highly ureolytic soil bacterium, revealed a mixed competitive and uncompetitive mechanism. The pH-dependence of the inhibition constants, investigated in the 6.5-8.0 range, reveals a predominant uncompetitive mechanism that increases by increasing the pH, and a lesser competitive inhibition that increases by lowering the pH. Ten crystal structures of the enzyme were independently determined using five crystals of the and five crystals of the protein crystallised in the presence of fluoride. The analysis of these structures revealed the presence of , in terminal and bridging positions (both fluorides are colored in gold). .
Structural studies on ureases have revealed that the immediate environment around the two Ni(II) ions at the active site is conserved, as to induce a common mechanism of catalysis whose key step is the nucleophilic attack of the nickel-bridging hydroxide on the urea molecule bound to the bimetallic nickel cluster via O and N atoms (see static image below).
The present study consistently supports an interaction of fluoride with the nickel centres in the urease active site in which (colored in salmon) to the Ni(II) ion proposed to coordinate urea in the initial step of the catalytic mechanism, while (colored in cyan) the Ni(II)-bridging hydroxide, blocking its nucleophilic attack on urea.
3D structures of urease
Urease 3D structures