User:Alexandra Gredová
From Proteopedia
- Full Real Name:
Alexandra Gredová
- Position:
Student
- Institution (NO ABBREVIATIONS):
Charles University in Prague, Faculty of Science
- City, State/Province, Country:
Prague, Czech Republic
- Field of Expertise or Study:
Master student in cell biology
Phenylalaninhydroxylase (PAH) mutations
Tereza Kovářová, Alexandra Gredová
Introduction
Phenylketonuria (PKU) is a human inherited autosomal recessive disorder, which constitute the most common inborn error of amino acid metabolism (1),(2). It is caused by variations in both alleles of a gene, which encodes for phenylalanine hydroxylase (PAH, phenylalanine 4-monooxygenase,EC 1.14.16.1), PAH gene (3). Phenylketonuria is most often caused by structural changes in phenylalanine hydroxylase (PAH), the enzyme that converts L-phenylalanine (L-Phe) to L-tyrosine(L-Tyr), resulting in accumulation of L-Phe to neurotoxic levels (4). Global prevalence of PKU is 1:24 000 live births and in Czech Republic it is about 1:5 500 live births (5),(6).
Gene structure
The human phenylalanine hydroxylase gene is located on human chromosome 12q23.2 locus (2). The PAH gene is comprised of 13 exons spanning 90 kb which represents about 2,9 % genomic sequence of this gene (7),(8). Length of exon varies within the gene and the shortest and longest exon is 57 bp and 892 bp, respectively (8). It is highly tissue specifically expressed in human, mainly in liver, kidney and gallbladder, as RNA-seq data show. This gene can be also known as PH, PKU, PKU1 (9).
Protein structure
|
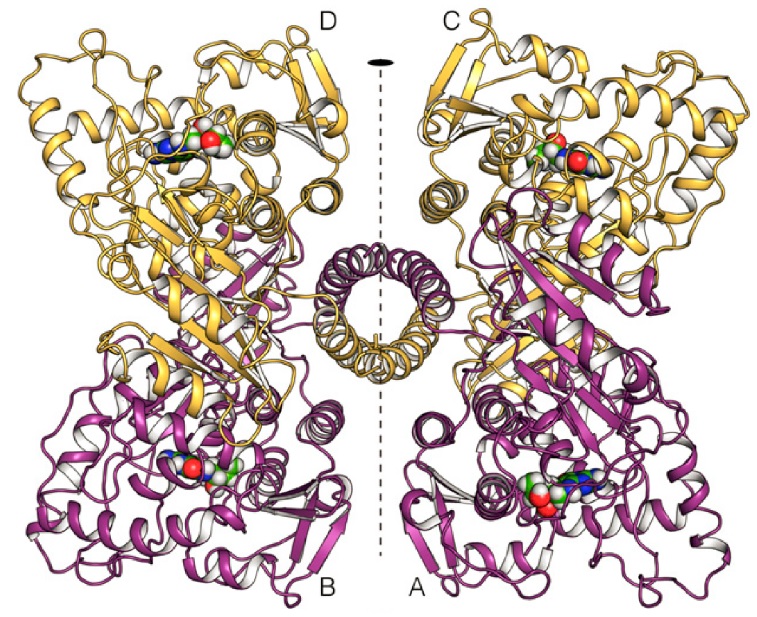
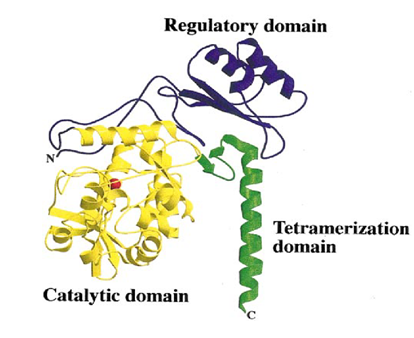
The human PAH enzyme occurs in the cell mostly as a homo-tetrameric enzyme of 52 kDa subunits (Fig. 1.) (10). The individual monomers (Fig. 2.) consist of an N-terminal regulatory domain (1-142), a central catalytic domain (143-410), and an oligomerization domain at the C-terminus (411-452) (11),(12). The regulatory domain has a common α-β sandwich motif, consisting of a four-stranded antiparallel β-sheets flanked on one side by two short α-helices and on the other side by the catalytic domain (12). The regulatory domain is necessary for manifestation of the regulatory properties, such as activation by L-Phe (13). The catalytic domain consists of 13 α-helices and 8 β-sheets (12). This domain contains the binding sites for iron, cofactor tetrahydrobiopterin (BH4), and substrate. At the active site iron binds to two histidines (His285 and His290) and a glutamate (Glu330) (13). The shortest domain, tetramerization domain resides on the C-terminus of PAH. It consists of two anti-parallel β-sheets forming a beta ribbon and one alpha helix. This domain is responsible for the connecting of 4 monomers into homo-tetramere. The α-helices from this oligomerizating domain cross the axis of symmetry of the tetramer and are interconnected into a coiled coil structure. Two rotation points within C-terminus have been discovered. At a position of Thr427 the chain is disordered, leading to its flexibility and bending of the tetramerization domain from the catalytic domain by 22 °, and at a position of Gly442 where the α-helix is bending by 20 ° (14). Flexibility of these regions could contribute to the right orientation of monomers and conformation of homo-tetramere (11)(14). This oligomeric and multidomain arrangement allows the complex regulation of PAH activity, including activation by the substrate L-Phe, which elicits a positive cooperative response on PAH, ensuring that neurotoxic accumulation of L-Phe is avoided, and decreasing PAH activity at low substrate concentration to preserve a lower threshold value of L-Phe for protein synthesis (10).
Function
PAH is an substrate specific enzyme from aromatic amino acid hydroxylase family (together with tyrosine hydroxylase and tryptophan hydroxylase). It converts L-phenylalanine to L-tyrosine by adding –OH group to an aromatic ring, which is a rate limiting reaction in L-Phe metabolism (15). PAH catalyzes the hydroxylation of its substrate by incorporation of O2 into the aromatic ring, and the final reaction also includes the reduction of the second O2 to H2O using two electrons supplied by cofactor BH4 (13). The activity is tightly regulated by a variety of mechanisms, including activation by the substrate phenylalanine, inhibition by the cofactor BH4, and additional activation by phosphorylation (16). In human cells, L-Phe as well as L-Tyr (and other aminoacids) enter brain after binding to large neutral amino acid transporter (LAT1) (3). Because L-Tyr is a precursor of catecholamines (important neurotransmiters and hormones), its defect in brain can cause neurophysiological changes. Transport of these aminoacids is also important for protein synthesis in the brain (13). Higher concentrations of L-Phe could lead to saturation of LAT1 and L-Phe could compete with transport of other aminoacids into brain (3),(10).
PAH mutations
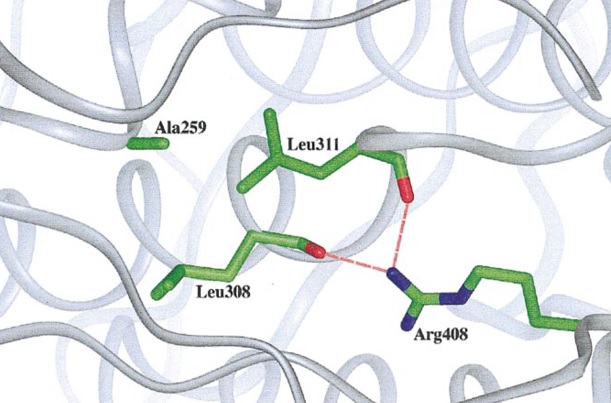
In the PAH gene have been identified 548 mutations, including missense, nonsense, insertion, deletion, frameshift and splicing mutations (16). Structurally, the mutations causing PKU can be shown to affect residues in five categories: active-site residues, structural residues, interdomain interactions in a monomer, residues interacting with the N-terminal autoregulatory sequence, and residues in the dimer or tetramer interfaces, mainly occurring in the catalytic and regulatory domains in exons 6, 7 and 11 (12),(17). The mutations result in a non-functional PAH that cannot participate in the L-Phe catabolism pathway. According to the BIOPKU database (database of patients with PKU, where information on genotype and phenotype can be found) from 2014, most common genotype occurring in the population is p.[Arg408Trp]; [Arg408Trp] (17). These patients have substitution CGG-> TGG in exon 12, which corresponds to the catalytic domain (14),(18). Structural studies indicate that this Arg408 is located on the loop between the tetramerization domain and the core monomer. Arg408 forms hydrogen bonds with Leu308 and Leu11 from helix 8 (Fig. 3.). These interactions are likely to stabilize the monomer structure and thus allow the correct orientation of the tetramerization domain, which is important for oligomerization (14).
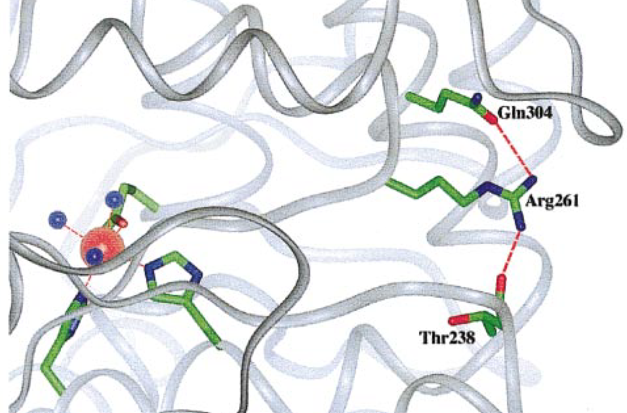
Substitution CGA-> TGA is another common mutation which ocurres in population in high frequencies. A genotype p.[Arg261Gln];[Arg261Gln] causes formation of premature STOP codon and truncated non-functional PAH protein is formed (Fig. 4.).
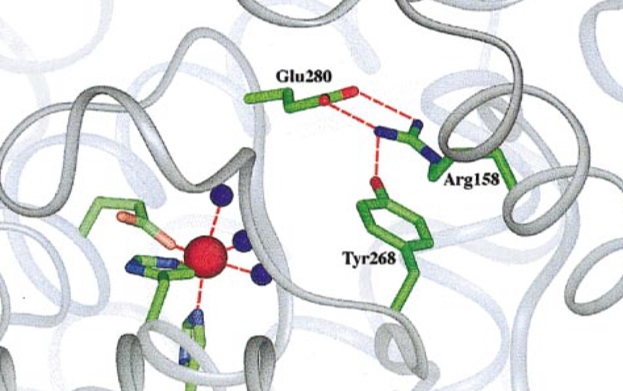
On the contrary substitution CGG-> TGG causes p.[Arg158Gln],[Arg408Trp] genotype where hydrogen bond between Arg158 and Tyr268 can´t be formed (Fig. 5.). This bond is important for forming a functional catalytic centre of the PAH enzyme in normal conditions (12),(17).
Phenylketonuria phenotype and clinical manifestations
Phenylketonuria is a metabolic disorder that results in intolerance to the dietary intake of the essential amino acid phenylalanine (7). Patients suffering from PKU have elevated blood concentration of L-Phe, more than 1200 μmol/L (normal concentration of L-Phe in blood is around 50-100 μmol/L). Patients following a strict diet won´t show no signs of illness and can live a normal life under constant control of the level of L-Phe in the blood (3). However, in the case of untreated PKU, there can be permanent consequences, such as mental disabilities, seizures, cognitive disorders, or milder forms that cause the typical musty odor, and hypopigmentation (1). There is a noticeable difference between the incidence of symptoms in well and poorly treated individuals (3).
Diagnostics
Because brain damage is irreversible, PKU (along with other hereditary diseases) is being identified by neonatal screening (NBS) to prevent poor development. NBS is based on bacterial inhibition assay for L-Phe using blood samples collected on filter paper cards to accurately measure L-Phe concentration (19).
Treatment
There is currently no cure for PKU (20). Dietary restriction remains a key therapy for this disorder (4). Patients must consume foods with reduced amino acid content, especially L-Phe. This diet is recommended from newborns to prevent poor brain development. Another possible therapy for adult patients whose diet is not as restricted in L-Phe is supplementation of LNAAs which decreases L-Phe concentrations in brain (20). Additional treatment strategies for PKU are currently being developed, in particular gene and RNA therapy (1).
References
(1) Van Spronsen, F. J. (2010) ‘Phenylketonuria: A 21 st century perspective’, Nature Reviews Endocrinology. Nature Publishing Group, 6(9), pp. 509–514. doi: 10.1038/nrendo.2010.125.
(2) Jaffe, E. K. (2017) ‘New protein structures provide an updated understanding of phenylketonuria’, Molecular Genetics and Metabolism. Elsevier Inc., 121(4), pp. 289–296. doi: 10.1016/j.ymgme.2017.06.005.
(3) Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010 Oct 23;376(9750):1417-27. doi: 10.1016/S0140-6736(10)60961-0. PMID: 20971365.
(4) Lichter-Konecki, U. and Vockley, J. (2019) ‘Phenylketonuria: Current Treatments and Future Developments’, Drugs. Springer International Publishing, 79(5), pp. 495–500. doi: 10.1007/s40265-019-01079-z.
(5) Alicia Hillert, Yair Anikster, Amaya Belanger-Quintana, Alberto Burlina, Barbara K. Burton, Carla Carducci, Ana E. Chiesa, John Christodoulou, Maja Đorđević, Lourdes R. Desviat, Aviva Eliyahu, Roeland A.F. Evers, Lena Fajkusova, François Feillet, Pedro E. Bonfim-Freitas, Maria Giżewska, Polina Gundorova, Daniela Karall, Katya Kneller, Sergey I. Kutsev, Vincenzo Leuzzi, Harvey L. Levy, Uta Lichter-Konecki, Ania C. Muntau, Fares Namour, Mariusz Oltarzewski, Andrea Paras, Belen Perez, Emil Polak, Alexander V. Polyakov, Francesco Porta, Marianne Rohrbach, Sabine Scholl-Bürgi, Norma Spécola, Maja Stojiljković, Nan Shen, Luiz C. Santana-da Silva, Anastasia Skouma, Francjan van Spronsen, Vera Stoppioni, Beat Thöny, Friedrich K. Trefz, Jerry Vockley, Youngguo Yu, Johannes Zschocke, Georg F. Hoffmann, Sven F. Garbade, Nenad Blau, The Genetic Landscape and Epidemiology of Phenylketonuria, The American Journal of Human Genetics, Volume 107, Issue 2, 2020, Pages 234-250, ISSN 0002-9297, https://doi.org/10.1016/j.ajhg.2020.06.006.
(6) David J, Chrastina P, Pešková K, Kožich V, Friedecký D, Adam T, Hlídková E, Vinohradská H, Novotná D, Hedelová M, Al Taji E, Holubová A, Skalická V, Macek M, Gaillyová R, Votava F. Epidemiology of rare diseases detected by newborn screening in the Czech Republic. Cent Eur J Public Health. 2019 Jun;27(2):153-159. doi: 10.21101/cejph.a5441. PMID: 31241292.
(7) Mitchell, J. J., Trakadis, Y. J. and Scriver, C. R. (2011) ‘Genetics in Medicine Phenylalanine hydroxylase deficiency’, 13(8). doi: 10.1097/GIM.0b013e3182141b48.
(8) Scriver CR. The PAH gene, phenylketonuria, and a paradigm shift. Hum Mutat. 2007 Sep;28(9):831-45. doi: 10.1002/humu.20526. PMID: 17443661.
(9) Fagerberg, L., Hallström, B. M., Oksvold, P., Kampf, C., Djureinovic, D., Odeberg, J., … Uhlén, M. (2013). Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Molecular & Cellular Proteomics, 13(2), 397–406. doi:10.1074/mcp.m113.035600
(10) Flydal, M. I. et al. (2019) ‘Structure of full-length human phenylalanine hydroxylase in complex with tetrahydrobiopterin’, Proceedings of the National Academy of Sciences of the United States of America, 166(23), pp. 11229–11234. doi: 10.1073/pnas.1902639116.
(11) Flatmark T, Stevens RC. Structural Insight into the Aromatic Amino Acid Hydroxylases and Their Disease-Related Mutant Forms. Chem Rev. 1999 Aug 11;99(8):2137-2160. doi: 10.1021/cr980450y. PMID: 11849022.
(12) Erlandsen H, Stevens RC. The structural basis of phenylketonuria. Mol Genet Metab. 1999 Oct;68(2):103-25. doi: 10.1006/mgme.1999.2922. PMID: 10527663.
(13) Flydal, M. I. and Martinez, A. (2013) ‘Phenylalanine hydroxylase: Function, structure, and regulation’, IUBMB Life, 65(4), pp. 341–349. doi: 10.1002/iub.1150.
(14) Fusetti F, Erlandsen H, Flatmark T, Stevens RC. Structure of tetrameric human phenylalanine hydroxylase and its implications for phenylketonuria. J Biol Chem. 1998 Jul 3;273(27):16962-7. doi: 10.1074/jbc.273.27.16962. PMID: 9642259.
(15) Kappock TJ, Caradonna JP. Pterin-Dependent Amino Acid Hydroxylases. Chem Rev. 1996 Nov 7;96(7):2659-2756. doi: 10.1021/cr9402034. PMID: 11848840.
(16) Jennings, I. G., Cotton, R. G. H. and Kobe, B. (2000) ‘Structural interpretation of mutations in phenylalanine hydroxylase protein aids in identifying genotype-phenotype correlations in phenylketonuria’, European Journal of Human Genetics, 8(9), pp. 683–696. doi: 10.1038/sj.ejhg.5200518.
(17) Blau N, Shen N, Carducci C. Molecular genetics and diagnosis of phenylketonuria: state of the art. Expert Rev Mol Diagn. 2014 Jul;14(6):655-71. doi: 10.1586/14737159.2014.923760. Epub 2014 May 31. PMID: 24882081
(18) DiLella AG, Marvit J, Brayton K, Woo SL. An amino-acid substitution involved in phenylketonuria is in linkage disequilibrium with DNA haplotype 2. Nature. 1987 May 28-Jun 3;327(6120):333-6. doi: 10.1038/327333a0. PMID: 2884570.
(19) Vockley, J. et al. (2014) ‘Phenylalanine hydroxylase deficiency: Diagnosis and management guideline’, Genetics in Medicine, 16(2), pp. 188–200. doi: 10.1038/gim.2013.157.
(20) Al Hafid N, Christodoulou J. Phenylketonuria: a review of current and future treatments. Transl Pediatr. 2015 Oct;4(4):304-17. doi: 10.3978/j.issn.2224-4336.2015.10.07. PMID: 26835392; PMCID: PMC4728993