User:Anielle Salviano de Almeida Ferrari/Sandbox 1
From Proteopedia
Contents |
Leptospira cAMP-dependent DGC 1 (Lcd1)
|
The leptospirosis is caused by spirochaetes of pathogenic species of genus Leptospira and is a emerging zoonotic disease worldwide [1] [2].According to the World Health Organization (WHO), approximately 1 million people become infected with leptospiras per year and 60,000 die from the disease worldwide [3].The ability of some Leptospira species to grow in different conditions, such as in soil, water and in different mammalian organs and in the bloodstream, suggests that the bacteria must have different signaling pathways to detect the external environment and modulate its gene expression. One way to respond to environmental changes and modulate gene expression is through the production of bis (3 ′, 5 ′) - cyclic diguanylic acid, known as cyclic di-GMP (c-di-GMP) [1]. However, to date, almost nothing is known about c-di-GMP signaling in Leptospira[1] [4][5][6].
Function
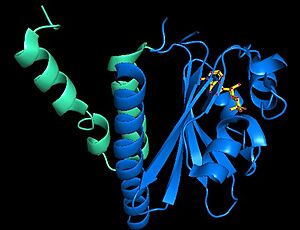
is coded by the LIC_13137 encoded a diguanylate cyclases that produced c-di-GMP in L. interrogans serovar Copenhageni strain Fiocruz L1-130. Lcd1 has two domains: the N-terminal GAF domain (residues 26-165) and the C-terminal GGDEF domain (residues 178-326) with a linker between them (residues 166-177)[1]. C-di-GMP is responsible for several behaviors in bacteria, such as biofilm, cell cycle, motility, chemotaxis, quorum sensing, virulence, and may the action of regular riboswitches [7]. Despite the efforts of different mechanisms of the immune system to eliminate infection by leptospires, these bacteria are able to evade the immune system and survive. A possible evasion mechanism is the production of biofilms, which helps bacteria to organize a physical-chemical barrier against the immune system and against antimicrobial agents [8].
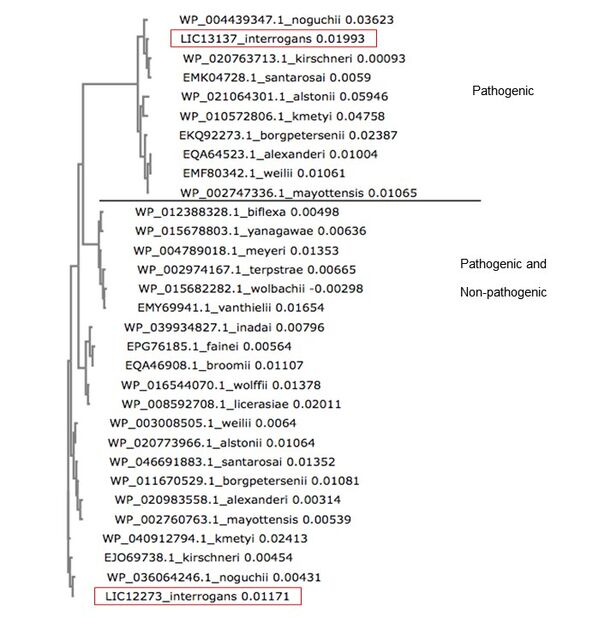
The gene LIC13137 has orthologs only in pathogenic species of Leptospira, with >23% identity. But, LIC13137 has only paralog, LIC12273, with the same architecture domain (GAF-GGDEF domain), founded in pathogenic and non-pathogenic species of Leptospira. Therefore, an elucidation of the production of Lcd1 by L. interrogans helps to understand the bacteria's virulence.
Structure of Lcd1
The fragment contained the GAF domain (residues 1 – 180) was purified and used in the thermal denaturation experiments in the presence of various cyclic nucleotides. In the addition of 3′,5′-cyclic adenosine monophosphate (cAMP) increases the thermal stability, despites the other ligands tested 3′,5′-cyclic guanosine monophosphate (cGMP) and c-di-GMP do not alter significantly [1]. The structure deposited in PDB bank was was obtained by crystallizing the GAF domain (residues 1 – 175), called Lcd1-GAF. In the assymetric unit, there are four Lcd1-GAF molecules. But using PISA algoritm for analysis to protein-protein interaction and size-exclusion chromatography (SEC) analysis of apo and holo state of Lcd1-GAF reveals that Lcd1 exists has a dimer. The structure of the Lcd1-GAF monomer is composed by three-helix bundle (α1 - α2 – α3), six antiparallel strands (β3 – β2 – β1 – β6 – β5 – β4), and more four α-helices, three small (α4 – α5 – α6) and a one 310 helix (α310).
The domain GAF is divided by two : the dimerization domain (DD) in and the ligand binding subdomain (BD).
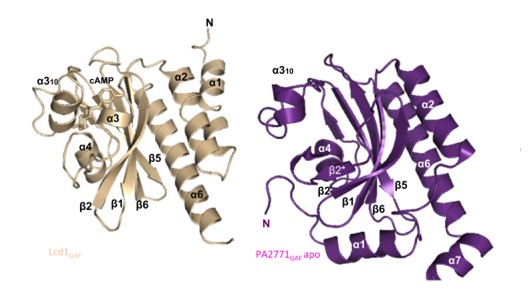
Structural comparison with PA2771, a full-length GAF-GGDEF structure
|
The only full-lenght GAF-GGDEF protein structure similar to Lcd1 is PA2771 from Pseudomonas aeruginosa, which GAF and GGDEF domains share 16% and 29% of similarity with Lcd1, respectively. Superposition of the structures of the Lcd1GAF–cAMP complex and the apo form of PA2771 (no ligand is recognized) revealed very similar ligand binding subdomain architectures, with the main differences being small shifts in the β5-β6 loop, β1 and β2 strands, and in the 310 helix. Also, PA2771 has an extra beta-strand (here was referred to as β2*) instead of α3 [1] [9].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 da Costa Vasconcelos FN, Maciel NK, Favaro DC, de Oliveira LC, Barbosa AS, Salinas RK, de Souza RF, Farah CS, Guzzo CR. Structural and Enzymatic Characterization of a cAMP-Dependent Diguanylate Cyclase from Pathogenic Leptospira Species. J Mol Biol. 2017 Jul 21;429(15):2337-2352. doi: 10.1016/j.jmb.2017.06.002. Epub, 2017 Jun 7. PMID:28601495 doi:http://dx.doi.org/10.1016/j.jmb.2017.06.002
- ↑ Vincent AT, Schiettekatte O, Goarant C, Neela VK, Bernet E, Thibeaux R, Ismail N, Mohd Khalid MKN, Amran F, Masuzawa T, Nakao R, Amara Korba A, Bourhy P, Veyrier FJ, Picardeau M. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl Trop Dis. 2019 May 23;13(5):e0007270. doi:, 10.1371/journal.pntd.0007270. eCollection 2019 May. PMID:31120895 doi:http://dx.doi.org/10.1371/journal.pntd.0007270
- ↑ Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015 Sep 17;9(9):e0003898. doi: 10.1371/journal.pntd.0003898., eCollection 2015. PMID:26379143 doi:http://dx.doi.org/10.1371/journal.pntd.0003898
- ↑ Xiao G, Kong L, Che R, Yi Y, Zhang Q, Yan J, Lin X. Identification and Characterization of c-di-GMP Metabolic Enzymes of Leptospira interrogans and c-di-GMP Fluctuations After Thermal Shift and Infection. Front Microbiol. 2018 Apr 20;9:764. doi: 10.3389/fmicb.2018.00764. eCollection, 2018. PMID:29755425 doi:http://dx.doi.org/10.3389/fmicb.2018.00764
- ↑ Thibeaux R, Soupe-Gilbert ME, Kainiu M, Girault D, Bierque E, Fernandes J, Bahre H, Douyere A, Eskenazi N, Vinh J, Picardeau M, Goarant C. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. NPJ Biofilms Microbiomes. 2020 Jun 12;6(1):24. doi: 10.1038/s41522-020-0134-1. PMID:32532998 doi:http://dx.doi.org/10.1038/s41522-020-0134-1
- ↑ Teixeira RD, Holzschuh F, Schirmer T. Activation mechanism of a small prototypic Rec-GGDEF diguanylate cyclase. Nat Commun. 2021 Apr 12;12(1):2162. doi: 10.1038/s41467-021-22492-7. PMID:33846343 doi:http://dx.doi.org/10.1038/s41467-021-22492-7
- ↑ Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013 Mar;77(1):1-52. doi: 10.1128/MMBR.00043-12. PMID:23471616 doi:http://dx.doi.org/10.1128/MMBR.00043-12
- ↑ Brihuega B, Samartino L, Auteri C, Venzano A, Caimi K. In vivo cell aggregations of a recent swine biofilm-forming isolate of Leptospira interrogans strain from Argentina. Rev Argent Microbiol. 2012 Jul-Sep;44(3):138-43. PMID:23102459
- ↑ Chen Y, Liu S, Liu C, Huang Y, Chi K, Su T, Zhu D, Peng J, Xia Z, He J, Xu S, Hu W, Gu L. Dcsbis (PA2771) from Pseudomonas aeruginosa is a highly active diguanylate cyclase with unique activity regulation. Sci Rep. 2016 Jul 8;6:29499. doi: 10.1038/srep29499. PMID:27388857 doi:http://dx.doi.org/10.1038/srep29499