User:Anjali Rabindran/Sandbox 1
From Proteopedia
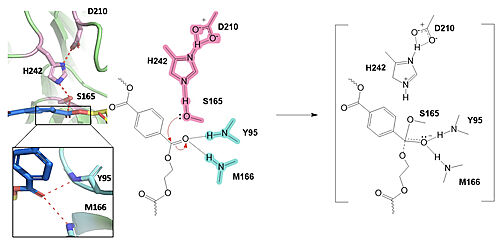
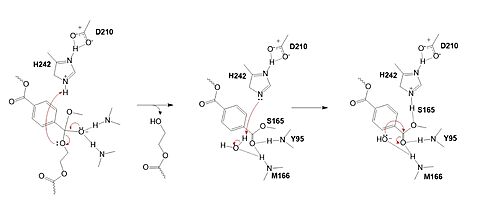
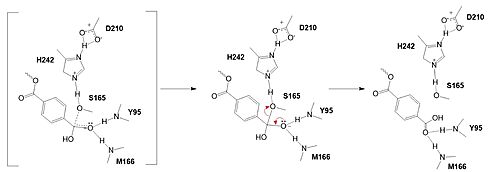
MechanismPolyethylene terephthalate (PET) hydrolase (PETase) is a serine hydrolase that catalyzes the cleavage of ester bonds in PET polymers. In the first step of the reaction, the nucleophilic serine residue (S165) attacks the carbonyl carbon of the PET substrate, forming a tetrahedral intermediate. The oxyanion hole stabilizes the negative charge. The mechanism of the PET Hydrolase involves a proton relay by the , making the catalytic S165 a good nucleophile. S165 attacks the carbonyl carbon in the -1 monomer of the PET Polymer, forming a tetrahedral intermediate. The pi electrons move onto the carbonyl oxygen, creating an oxyanion, which is stabilized by the , consisting of the backbone amide nitrogens of Y95 and M166. Then, the leaving group oxygen on the -2 monomer is protonated by H242, which is also a part of the catalytic triad. This facilitates the reformation of the carbonyl group upon collapse of the oxyanion and the severing of the . In the final step, the water-activated nucleophile attacks the carbonyl carbon of S165, generating a second tetrahedral intermediate. The collapse of this intermediate regenerates the carbonyl double bond and removes the catalytic serine residue. The oxyanion hole residues stabilize the negative charge of this transition state, allowing the -1 monomer to be released from the enzyme and the protons to reset for further catalysis. MutationsResearchers have been investigating various mutations of PET hydrolase to enhance its catalytic ability. One group of researchers, Tournier et. al., have made mutations in the PET hydrolase active site. They identified the key residues involved in the catalytic mechanism by using a model of the (2-HE(MHET)₃) onto the enzyme (PDB ID 4EB0). The site, mainly a hydrophobic pocket, contained 11 residues targeted for mutagenesis. From this, they identified that the majority of enzymes' specific activity went down; however, the mutation of the to either isoleucine or tryptophan increased specific activity. Four target mutations introduced into the PET Hydrolase by Tournier et. al. demonstrated improved catalytic efficiency and thermal stability compared to its wild-type structure. F243W MutationThe mutation of the F243 position to a tryptophan () was selected for further analysis based on its enhanced catalytic activity in the depolymerization of Pf-PET. The W243 mutation was shown to improve the substrate's binding affinity and increase the enzyme’s activity compared to the wild-type enzyme. This was one of the few variants that exhibited higher activity, and it was further analyzed through differential scanning fluorimetry (DSF) to assess its stability. [1] F243I MutationSimilar to the W243 mutation, the was identified as a variant with improved depolymerization activity of Pf-PET. The I243 mutation in LCC led to better substrate interaction than the wild-type enzyme. After generating all possible variants, this mutation was among the few that exhibited 75% or more of the wild-type specific activity. As with the W243 mutation, DSF was used to determine the melting temperature and thermal stability, supporting the increased activity observed with this mutation. [1] Specific Activity ResultsTable 2. Specific activity and rate of wild-type, WCCG, and ICCG mutants. Initial rate was measured by the calculated rate of reaction by NaOH consumption. Specific activity was measured via pf-PET-depolymerization assay.[1]
Both the WCCG and ICCG mutants display slightly higher initial rates (30.3 and 31.0 g hydrolyzed PET•L⁻¹•h⁻¹, respectively) compared to the wild-type enzyme (25.7 g hydrolyzed PET•L⁻¹•h⁻¹). This suggests that the mutations introduced in WCCG and ICCG enhance the rate of PET breakdown. The specific activity of the WCCG mutant (75.9 mg TAeq•h⁻¹•mg⁻¹ enzyme) is slightly lower than that of wild-type PETase (81.9 mg TAeq•h⁻¹•mg⁻¹ enzyme), whereas the ICCG mutant shows comparable specific activity to the wild-type (82.0 mg TAeq•h⁻¹•mg⁻¹ enzyme). Mutating the active site F243 to I243 (in the ICCG mutant) and W243 (in the WCCG mutant) resulted in a percent gain of specific activity of 127.5% and 118.4% respectively.[1]
References
| ||||||||||||||||||||||||