User:Brenda Bott/Sandbox1
From Proteopedia
|
Rubisco Structure
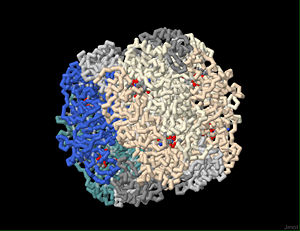
Across all organisms, rubisco enzymes consist of more than one subunit. There are two structural forms of based on number of subunits. The simplest rubisco (form II) is found in some photosynthetic bacteria, and is a dimer of two large subunits (L), each of which is composed of approximately 475 residues. Form I is the more common type of rubisco. It is composed of two different sized subunits, small (S) and large (L). Large subunits of form I are, like those of form II, approximately 475 residues, and small subunits are approximately 120 residues in length. Form I rubisco is a hexadecamer, made of eight L and eight S subunits (8L8S). All eight L subunits are identical to each other and are arranged in the hexadecamer complex as four dimers around a central axis. This octameric core is roughly spherical; a cluster of four S subunits forms a cap on both ends of the sphere. The L subunit is roughly pear-shaped in all forms of rubisco, The narrower, amino-terminal end of the L subunit is composed of the first 150 residues. The larger, carboxy-terminal end is built of residues 151 to 475, and consists of an alpha / beta barrel structure made of eight alpha helices and eight beta-strands.
The Active Site
Each large subunit of all forms of rubisco has one substrate-binding site which becomes an intact active site with the head-to-toe, antiparallel association of the coordinating large subunit in the dimer (Anderson and Backlund, 2008). The formation of a dimer is necessary to complete two active sites, with each large subunit providing opposite halves of the active site to the other. Thus, there are two active sites per dimer, and eight per rubisco complex. The active site of the enzyme is found at the interface of the L subunits in each dimer; the active site is constructed of elements from both subunits. Most of the active site residues are contributed by the loops connecting alpha-helices with beta-strands at the carboxy-terminal end of the alpha/beta barrel structure in the carboxy-terminal domain (wide end) of one of the L subunits of the dimer. However, two loops in the amino-terminal domain (narrow end) of the second L-subunit contribute additional residues to the active site. The entrance to each active site faces the outer surface of the hexadecameric molecule. The S subunits do not contribute directly to the active site, but interactions between L and S subunits affect enzyme function and CO2/O2 specificity (Andersson & Backlund, 2008).