User:Brittany A. Gabriel
From Proteopedia
| This Sandbox is Reserved from 13/03/2012, through 01/06/2012 for use in the course "Proteins and Molecular Mechanisms" taught by Robert B. Rose at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 451 through Sandbox Reserved 500. | |||||||
To get started:
More help: Help:Editing For more help, look at this link: http://www.proteopedia.org/wiki/index.php/Help:Getting_Started_in_Proteopedia
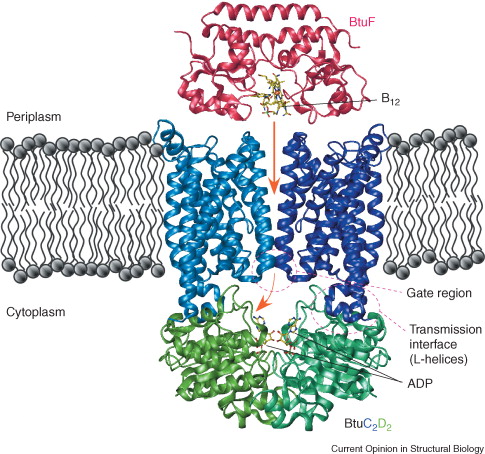
Vitamin B12 Binding ProteinVitamin B12-binding protein, also referred to as its gene name BtuF, is a part of the ABC transporter complex, BtuCDF. This complex is what is involved in the importation of the actual vitamin B12. The overall function of this protein is to bind the vitamin B12 and deliver it to the periplasmic surface of the ABC transporter BtuCD [1]. The organism in which this protein is derived from is Escherichia coli (strain K12). This organism is widely used as a model organism in many research efforts to date. Btuf belongs to a subclass of periplasmic binding proteins (PBPs)
StructureThe 28kDa Vitamin B12-binding protein contains two α/β domains, which are linked by a rigid α-helix that spans the length of the protein. Each domain consist of a central five-stranded β-sheet surrounded by helices. It resembles the ferrichrome binding protein FhuD by its bi-lobed fold. The vitamin conformation, or “base-on” conformation is where the bound vitamin B12 can be found within the deep acidic cleft formed at the interface between the two BtuF lobes [2]. It is bound by the enzymes methionine synthase, methylmalonyl-coA mutase, and glutamate mutase. Iron and b12 uptake are mediated by the conservation among binding proteins and ABC transporters of two surface glutamates from BtuF that interact with arginine residues on the periplasmic surface of the BtuCD transporter. This is thought to play a role in docking and the transmission of conformational changes [3]. Ligand ActivityWithin the enzymes mentioned above,the pseudonucleotide tail of B12 is extended and the nitrogen of a nearby histidine side chain is able to act as an axial ligand to the cobalt. The "base-on" conformation of the BtuF-bound B12 allows the N3B nitrogen of the dimethylbenzimidazole (DMB) base to serve as an axial ligand to the central cobalt atom. Secondary StructureThe BtuF protein possesses a typical cleaved N-terminal signal sequence that extends from residues 1-106. The C-terminal domain is structurally similar to the B12-binding domain that can be found in various enzymes. It includes residues 130-244 of the mature protein. Conformational change that may involve an increase in domain mobility is said to be a result of unwinding of an α-helix located in the C-terminal domain. This unwinding would occur upon the binding on BtuF with BtuC and would thus result in the release into the transport cavity. Overall, it is the free mobility of the C-terminal domain that is essential to the release of BtuF necessary for the completeion of the transport cycle [4].
Methods for Structure AnalysisIn order to solve for the vitamin B12-binding protein, SDS-PAGE can be conducted. This method is effective considering that it selects for the site of signal cleavage within a molecule. It is the N-terminus of this protein, which contains the signal sequence site, that will be identified by this procedure.
Mechanism of ActionThe binding mechanism of BtuF with vitamin B12 is entropically driven. There are two residues located near the exterior of the binding pocket of the molecule. These pockets act as a gate in the binding and release of vitamin B12. When the vitamin B12 is bound, BtuF is normally in a closed, stable conformation. Two surface glutamates from BtuF are able to interact with arginine residues on the periplasmic surface of BtuCD [5].
Medical ImplicationsA specific vitamin B12-binding protein relevate to human life is Haptocorrin. It is present in high amounts in different body fluids including human milk. This protein tends to inhibit the growth of specific E. coli strains. Recent research efforts are determining whether the antibacterial properties of this protein may exert a general defense against pathogens. Its affect on the composition of the developing microbiota in the gastrointestinal tracts of breastfed infants are being researched as well [6].
References
|