User:Catherine Laun/Sandbox1 Myelin Oligodendrocyte Glycoprotein
From Proteopedia
|
Contents |
Introduction
Myelin Oligodendrocyte Glycoprotein (MOG) is a protein related to Multiple Sclerosis, a debilitating autoimmune disorder.
Multiple Sclerosis (MS) is a chronic and progressively degenerative autoimmune disorder in which the immune system actually attacks itself, resulting in an increasing loss in nerve function. The effects of MS can be small and gradual or severe and rapid depending on each individual case. The nerve damage associated with MS is caused by demyelination which is attack of the myelin sheath. The myelin sheath acts as a kind of insulator for the axon it surrounds (analogous to the rubber/plastic covering for the otherwise exposed wires of your electronics). The myelin sheath aids in nerve function by protecting the axon and sending the message faster.
Myelin oligodendrocyte glycoprotein is an integral membrane protein that is expressed exclusively by oligodendrocytes and myelin lamellae of the central nervous system (CNS). MOG is considered a minor protein component of the myelin sheath, making up a mere .05% of total myelin peptides (2). MOG expression is found only in mammals (1), studied primarily in mice and humans, and has been determined to be coded for by human chromosome 6p21.3-p22 and mouse chromosome 17 (3). MOG has been observed in both monomeric and dimeric forms (3). MOG acts as a cell-surface antigen expressed on the cells forming the myelin sheath. Like any other antigen, MOG is recognized by antibodies- in this case, anti-MOG antibodies. Such antibodies are produced when a humoral immune response against MOG has been activated. Such an autoimmune response is not typical; anti-MOG antibodies are normally not activated to recognize and attack MOG. This self-versus-self response has been suggested to be a contributing cause of MS.
What Is It?
|
Basic Structure
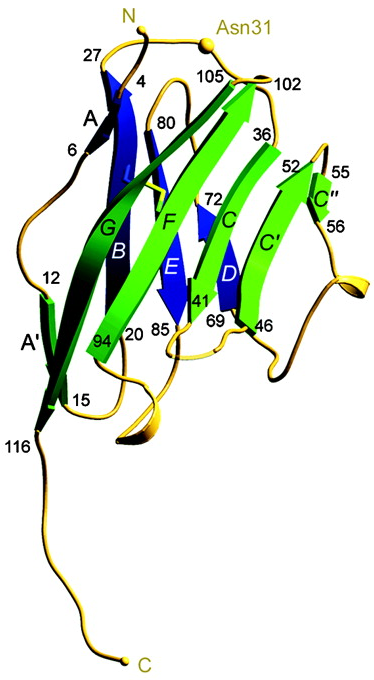
MOG is a 116 amino acid sequence (5), type I integral membrane protein with a single extracellular domain that adopts a classical IgV fold. Its amino acid sequence (primary structure) is (>90%) among animal species, most notably, mouse, rat, human, and bovine MOG (2). This conservation amongst species may be crucial for further studies into the structure and function of MOG and its relation to MS, potentially having vital importance in future trials or experiments. Most of the MOG consists of a compact, beta sandwich domain with one antiparallel beta-sheet () packed against a mixed beta-sheet () with N and C termini at opposite ends of the molecule (2). Also contributing to secondary structure are three alpha-helices.
Game-Changing Structural Elements
Myelin Oligodendrocyte Glycoprotein has three major structural components: three alpha helices, a beta sandwich domain consisting of two compact beta sheets (ABED and A'GFCC'C")(2), and an apparent binding/glycosylation marked by residue Asn-31. This site is key in binding the anti-MOG antibodies and consists of three loops, an (Phe-44), and a that runs about half the length of the protein (2) (and terminates in a short ). This electronegative strip is significant because it is typically indicative of ligand-binding interactions (2). One such ligand that has been shown to bind MOG in vitro is C1q, a component of complement capable of activating the complement pathway (1,2).
There are a variety of amino acids that are critical to the structure and resulting function of MOG. As stated above, Asn-31 serves as the binding site for anti-MOG antibodies. Phe-44 is a key exposed aromatic residue. is potentially important to the binding of anti-MOG antibodies as it protrudes from the polar, uncharged face of MOG formed by the , , and loops.
What Does It Do?
Major Function
Although the specific physiological function of MOG is unknown, it is likely that MOG acts as an adhesion and/or ligand-binding molecule (1). As mentioned above, MOG has been shown to bind C1q, a component of complement which is capable of initiating the complement cascade. Activation of the complement pathway results in the activation of an innate immune response. This is another possible function of MOG.
MOG also appears to have a role in the later stages of myelinogenesis (myelin-creation), especially the completion and maintenance of myelin (2). MOG is expressed late during brain development compared to other myelin proteins which speaks to the proposed role of MOG in late stages of myelination for the completion and maintenance of myelin integrity (3). MOG has been hypothesized to act as an adhesin in trans which means that it has the capacity to affect neighboring cells. If this is true, MOG can act as somewhat of a glue, holding together neighboring myelinated fibers in the CNS (which is a phenomenon observed in CNS, but not in the peripheral nervous system where MOG is not present) (2).
MOG and Multiple Sclerosis
Multiple Sclerosis is characterized by inflammation, demyelination, and progressive neurodegeneration (4). The lesions indicative of MS exacerbations are packed with T cells, B cells, and macrophages which immediately suggest a strong innate and adaptive response being mounted against an antigen in the body (4). As we have discussed already, it has been suggested that MOG is one such self-antigen that the body is mounting a defense against. The demyelination indicative of MS is strongly believed to be antibody-mediated, complement-dependent, and a response to proteins expressed by the oligodendrocytes that make up the myelin sheath, like MOG (4).
The interaction between antigen and targeted antibody (MOG and anti-MOG antibodies) causes destruction of myelin and axonal loss in experiments with animals. MOG is the only antigen that can induce a pathogenic demyelinating antibody response known as autoimmune encephalomyelitis, such as that seen in MS, in animals (1,4). In animals with autoimmune encephalomyelitis, anti-MOG antibodies, like those in humans, strongly enhance the T cell and macrophage-initiated demyelination thus increasing disease severity (4).
Being that demyelination and axonal loss are hallmarks of Multiple Sclerosis, it is strongly suggested that this interaction between MOG and anti-MOG antibodies could be a cause of the debilitating effects of MS. Patients with MS can fall into a variety of stages: primary progressive, secondary progressive, and relapsing-remitting. Each stage is characterized by different symptomology, progression, and as experiments have shown, different levels of anti-MOG antibodies. In fact, studies have shown that anti-MOG antibodies appear to predict the incidence of relapse in early MS patients (4). In human trials, it has been observed that in patients with primary progressive MS, there was a high quantity of anti-MOG antibodies (mostly IgG) (4). Scientists believe that the appearance of such antibodies, like 8-18C5 could be a good predictor for an MS exacerbation in recently diagnosed patients (4). Similarly, studies have also shown that anti-MOG antibodies might be a good predictor of early conversion to clinically definite MS during the early inflammatory stage of MS which can be very inconclusive (5). Given that MS is a very difficult disease to diagnose, the presence in large numbers of such antibodies might be used as a diagnostic tool for cases of suspected MS in the future (5).
Why Is It Important?
Implications
MOG forms a complex with an anti-MOG antibody, specifically Ab 8-18C5. This antibody specifically recognizes MOG and binds to the upper, membrane-distal surface of MOG where it interacts with BC, C'C", and FG loops (1). This interaction between MOG and Ab 8-18C5 results in demyelination in vitro and in vivo (1). The FG loop, in particular, is the dominant part of MOG that binds Ab 8-18C5 which makes it a key location to study for developing therapeutic strategies (1). If scientists were to somehow target the FG loop- anti-MOG antibody interaction and inhibit binding, the results could be groundbreaking for MS treatment.
Allow me to make my own hypothesis about the significance of MOG in the progression of MS symptomology. Autoimmunity to MOG could be related to the timing of MOG expression in development. MOG expression already occurs late in nervous system development, appearing to perform a critical job in finishing the construction of myelin and preserving its integrity. Perhaps, if MOG expression occurs even later in development than normal, the more-developed immune system recognizes MOG as a non-self antigen instead of a self antigen, thus triggering an autoimmune response to MOG. Late expression of MOG could also mean that the myelin sheath has been compromised, potentially causing inflammation and resulting in drawing fire from the immune system to the area of interest. Should an immune response then be targeted towards myelin, MOG (an innocent bystander) might be inappropriately targeted for destruction.
Future Research
Future research surrounding Myelin Oligodendrocyte Glycoprotein is likely to be focused on determining the specific physiological function of MOG and how anti-MOG antibodies become activated against MOG. Scientists in the field would also want to clarify the role of MOG and anti-MOG antibodies in the progression of Multiple Sclerosis, given its current controversial link. Should these aspects of MOG be elucidated and a clear link between MS and MOG be determined, scientists will have a better chance at developing more effective, targeted treatments for MS, and possibly a cure.
Another focus of future research might be to compare MOG to other closely related proteins whose function has been more clearly described. MOG has been determined to be most structurally similar to Sialoadhesin, although it still only shares about 24% amino acids (2). MOG has also been determined to be similar to b7-2, a receptor involved in cell adhesion and receptor-ligand interactions (2). These similarities to other known proteins suggests that scientists are correct in hypothesizing that MOG has a function in cell adhesion and ligand interactions. Perhaps future research might aim at using these related proteins as guides to help decode the specific physiological function of MOG and what goes wrong with that function in patients exhibiting MOG-atrributed demyelination.
There is a staggering amount of current research surrounding Multiple Sclerosis. There are many new clinical trials that will help to test new therapeutic strategies. There is also a significant amount of current research on the body's capacity for healing itself, and in this case, its potential capacity for repairing or replacing myelin that has been destroyed by autoimmune disorders like multiple sclerosis (6).
Many mysteries continue to surround the nature of Multiple Sclerosis and other such autoimmune disorders. The scientific community continues to work hard to elucidate some of the key components that keep us from creating better treatments or maybe even a cure. Given the amazing things that are being accomplished by innovative scientists each and every day, there is certainly hope that a future cure for MS is possible. For more information on the disease and the MS community, as well as ways you can help make a difference, visit The National MS Society.
References
(1)Breithaupt, C., Schubart, A., Zander, H., Skerra, A., Huber, R., Linington, C., Jacob, U. (2003). Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. The National Academy of Sciences of the USA, 100 (16), 9446-9451.
(2)Clements, C.S., Reid, H.H., Beddoe, T., Tynan, F.E., Perugini, M.A., Johns, T.G., Bernard, C.C.A, Rossjohn, J. (2003). The crystal structure of myelin oligodendrocyte, glycoprotein, a key autoantigen in multiple sclerosis. The National Academy of Sciences of the USA, 100 (19), 11059-11064
(3)Pham-Dinh, D., Mattei, M., Nussbaum, J., Roussel, G., Pontarotti, P., Roeckel, N., Mather, I.H., Artzt, K., Lindahl, K.F., Dautigny, A. (1993). Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. The National Academy of Sciences of the USA, 90, 7990-7994.
(4)Lalive, P.H., Menge, T., Delarasse, C., Gaspera, B.D., Pham-Dinh, D., Villoslada, P., von Budingen, H.C., Genain, C.P. (2006). Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. The National Academy of Sciences of the USA, 103 (7), 2280-2285.
(5)Zhou, D., Srivastava, R., Nessler, S., Grummel, V., Sommer, N., Bruck, W., Hartung, H., Stadelmann, C., Hemmer, B. (2006). Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. The National Academy of Sciences of the USA, 103 (50), 19057-19062.
(6)Redmond, S.A. & Chan, J.R. (2012). Revitalizing Remyelination- the Answer is Circulating. Science, 336, 161-162.