User:Daniel Parrell/Sandbox 1
From Proteopedia
Contents |
Aquaporin 4
Background
The discovery of aquaporin channels was a brakthrough discovery relating to water transport in cells. Peter Agre's discovery of the aquaporin channel family, answered a key question that had stumped biochmesists and cell biologists for years. This was the question of how is water abe to transport across the hydrophobic lipid bilayer so efficiently? When experimentally determined, the rate of water transport is able to occure at a rate of about 10^9 molecules per second, this is many times the limit set by diffusion across the membrane. There are 13 known mammalian aquaporins. Each is expressed in different tissues, and serve different functions based of the common theme of water balance. An advantage of this protein redundancy where an organism has different versions of one protein with a similar function in higher animals, is that it allows for specialized expression, and regulation of function to specific tissues. Aquaporins are an example of this phenomenon in mammals and other animals.
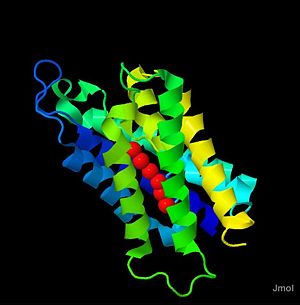
Aquaporin 4 (3GD8) is part of the aquaporin family. Majorly expressed in brain astrocytes, it contains the same structural and functional motifs typical of the aquaporin family. Aquaporin 4 (AQP-4) forms a complex in the membrane, composed of four AQP-4 monomers from the membrane bound to produce the tertiary structure. Aquaporin 4 (AQP-4) has been studied heavily in relation to the effects of brain swelling or injury caused by stroke, or trauma, and is largely implied as a regulator of homeostatic levels of water in the brain. Mutatnt mice for the homolouge of human AQP-4 have been developled and studied to gain knowlege on AQP-4 function in the body and the consequences of loss of function. To data the AQP-4 structure has been determined to 1.8 angstrom resolution, and the study of this structure gives helpful insight into the mechanism for water transport by this membrane channel.[1]
|
Structure
Hydrogen Bonding in the Channel
One of the defining features of aquaporins, and of Aquaporin 4 is the center ,its amino acids, and how it interacts, through hydrogen bonding,to drive water transport. The water molecules are held in the channel by the carboxyl oxygens of the amino acid Gly-93, Gly-94, His-95, and Ile-96, Gly-209, Ala-210, Ser-211, and Met-212. The spcific orientation of water in the channel is thought to be driven by a conserved sequence of amino acids called the (Asp-Pro-Ala) that is thought to hold the center water molecules in a specific orientation in the channel which defines the orientation of the other water molecules.
Water Selectivity
The aquaporins as a family are very selective for water, and not other solutes. In fact, most are completely specific for water, and only a few members of the family allow other solutes such as glycerol or urea to move through the channel.[1] The water selectivity of the channel is determined on the extracellular side by what is refered to as a "molecular filter". The selectivity for water on the extracellular side is achieved by reducing the channel diameter to a size that is selective for water molecules. This molecular filter is produced by the amino acids Arg-216 and His-201, and effectiviely blocks the transport of other molecules.[1]
The selectivity for water on the cytoplasmic side of the membrane is also determined by specific amino acid residues, and their interactions with water. On the cytoplasmic side of the channel, the amide backbones of Ser-188 and Gly-189 produce the interactions to produce wter selectivity. These amino acids along with the tetrameric tertiry structure of AQP-4 produce a stabilized arrangement of 4 water molecules that contribute to the selectivity for water. [1] This structure allows the channel to exclude other solvents using both charge based and spacial exclusion of other molecules. It is the selectifity of aquaporins, that makes them such a good part of the systems used by organisms to maintian osmotic homeostasis.
Implications in Heath and Science
Implications for Brain swelling, and mutant mice
Because of the localized expression of AQP-4 in brain astrocytes, the function of AQP-4 has been intensely studied in relation to diseases such as stroke, brain edema, and cranial swelling. Often trauma experienced by the brain by stroke for example, results in an increase in extracellular water in the brain (Pasantes-Morales and Cruz-Rangel 2010). The occurrence of cranial swelling is often counteracted and absorbed by systems such as the cerebrospinal fluid or the blood stream. When these systems cannot keep up with swelling, the pressure builds and the extracellular environment becomes hypotonic. A result of increased extracellular water in the brain is swelling of the astrocyte cells, which have been shown to highly express APQ-4(Pasantes-Morales and Cruz-Rangel 2010). This swelling is due to influx of water, most likely due to the action of AQP-4. Swelling of astrocyte cells is the last effort to maintain homeostatic water balance, the following event if this fails is cell death following the intake of too much water (Papadopoulos and Verkman 2007).
Mutant mice for human AQP-4 homologues have been discovered, and studied to determine the physiological response to AQP-4 knockout, and the response of these mice to cranial swelling. A common observation among these experiments was a decrease in the amount of water transport into astrocyte cells as well as into the brain extracellular environment (Papadopoulos and Verkman 2007). This can have good and bad effects, depending on the conditions behind the cranial swelling. When the excess water originates outside of the brain, it is effectively excluded, and cranial swelling is not observed (Papadopoulos and Verkman 2007). If the water makes it into the brain extracellular environment, it becomes difficult to lower the water level, and damage may occur (Papadopoulos and Verkman 2007).
AQP-4 and Bovine Spongiform Encephalopathy
Another area of research that has discovered a role for AQP-4 is in the study of Bovine Spongiform Encephalopathy (BSE). BSE is a protein disorder where a mutated prion protein precipitates and causes an aggregation of the prions, causing cellular dysfunction, and possibly cell death (Costa et al. 2007). The aggregation of prions in the cell causes an osmotic problem by making the cell hyperosmotic, and as result AQP-4 (and AQP-1) have been shown to be overexpresse in the brains of BSE infected cattle (Costa et al. 2007). This overexpression could be a biological reaction to the increase in osmotic stress caused by the prion aggregate. This overexpression, however, may also lead to an imbalance in water levels and consequent neuronal dysfunction, or cell death.
References
- ↑ 1.0 1.1 1.2 1.3 Ho, J., Yeh, R., Sandstrom, A., Chorny, I., Harries, W., Robbins, R., Miercke, L. and Stroud, R. 2009 Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance Proceding of the national academy of sciences 106.18:7437-7442.
Citations
1. Tani, K., Mitsima, T., Hiroaki, Y., Kamegawa, A., Nishikawa, K., Tanimura, Y., and Fujiyoshi, Y. 2009. Mechanism of Aquaporin-4's Fast and Highly Selective Water Conduction and Proton Exclusion. Journal of molecular and biochemistry 389:694-706
2. Pasantes-Morales, H. and Cruz-Rangel S. 2010. Brain volume regulation: osmolytes and aquaporin perspectives. Neuroscience 168:87-884.
3. Papadopoulos, M., and Verkman, A. 2007. Aquaporin-4 and brain edema. Pediatr Nephrol 22:778-784.
4. Costa, C., Tortosa, R., Rodriguez, A., Ferrer, I., Torres, J., Bassols, A. and Pumarola, M. 2007. Aquaporin 1 and aquaporin 4 overexpression in bovine spongiform encephalopathy in a transgenic murine model and in cattle field cases. Brain Research 11785:96-106.
5. Ishibashi, K., Hara, S. and Kondo, S. 2008. Aquaporin water channels in mamals. Clinical Experimental Nephrology 13.2:107-117.
6.
7. Nelson D., Cox, M. 2008. Aquaporins form hydrophilic transmembrane channels for the passage of water. In Lehninger Principles of Biochemistry, 5th ed. W.H. Freeman and Company. New York , NY. Pg.404-406.