PilZ domain
The concept of PilZ Domains as c-di-GMP Effectors is supported by compelling bioinformatics analysis. PilZ domains, typically located at the C-terminus of cellulose synthetase, produced by the bcsA gene, have been proposed to act as effectors for the signaling molecule cyclic diguanylate monophosphate (c-di-GMP). The discovery that c-di-GMP functions as an allosteric regulator of cellulose synthetase in Gluconacetobacter xylinus, as demonstrated by Benziman and colleagues, represented a significant breakthrough in the field.
Notably, the PilZ protein in Pseudomonas aeruginosa contains a single 118-residue domain exhibiting substantial sequence similarity to the C-terminus of BcsA. Within the pil operon, responsible for the synthesis and functionality of pili, PilZ is among the few proteins with an unknown function. This operon plays a role in the transition from motile to sessile growth modes, although the precise regulatory mechanisms remain elusive. Strains lacking the PilZ domain (ΔpilZ) can produce normal levels of pilin protomers but are unable to assemble functional pili.
Furthermore, other PilZ-domain proteins have been implicated in the regulation of motility across various organisms. For instance, the YcgR protein in Escherichia coli and the DgrA and DgrB proteins in Caulobacter crescentus have demonstrated involvement in controlling motility under high cytosolic c-di-GMP concentrations. Notably, certain PilZ domains are situated at the C-terminus of proteins participating in processes sensitive to c-di-GMP levels. Alg44 from P. aeruginosa represents another example, functioning as an essential protein in the c-di-GMP-regulated process of alginate biosynthesis. These discoveries provide robust evidence supporting the hypothesis that the majority of PilZ domains function as effectors, binding c-di-GMP, and exerting a critical role in the regulation of various cellular processes. [2] [3]
c-di-GMP
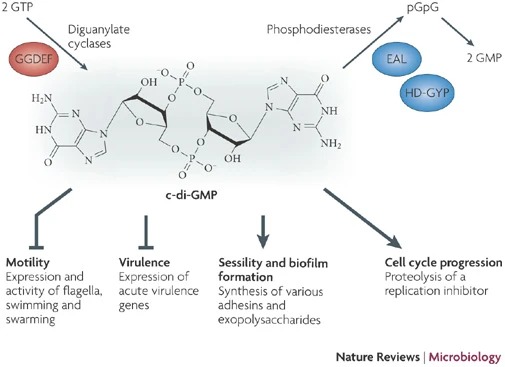
Cyclic dimeric guanosine monophosphate (c-di-GMP) was initially discovered by Benziman and colleagues as an allosteric activator of the membrane-bound cellulose synthase in Gluconacetobacter xylinus. It serves as a soluble molecule functioning as a second messenger in bacteria. Its role encompasses the stimulation of adhesin biosynthesis, exopolysaccharide matrix substance production in biofilms, and the inhibition of various forms of motility. This dynamic regulation controls the transition between planktonic motile behavior and the sedentary lifestyle associated with biofilm formation in bacteria (Figure 1). Furthermore, c-di-GMP governs virulence in animal and plant pathogens, cell cycle progression, antibiotic production, and other cellular functions. However, the precise molecular mechanisms underlying these processes and the specific targets affected by c-di-GMP-binding effector components are still being elucidated. [4]
The synthesis of c-di-GMP involves the conversion of two guanosine triphosphate (GTP) molecules by diguanylate cyclases (DGCs), while its breakdown yields 5′-phosphoguanylyl-(3′-5′)-guanosine (pGpG) through the action of specific phosphodiesterases (PDEs). pGpG is subsequently cleaved into two guanosine monophosphate (GMP) molecules (Figure 1). DGC activity is associated with the GGDEF domain, named after the crucial amino acid sequence motif found in the enzyme's active site. On the other hand, c-di-GMP-specific PDE activity is linked to the EAL or HD-GYP domains, with their respective amino acid motifs playing essential roles in their enzymatic functions.
The significance of c-di-GMP regulatory systems gained considerable attention following the advent of whole genome sequencing, revealing the widespread presence of GGDEF and EAL domains in bacterial species, often with a large number of encoded proteins. Artificial modulation of cellular c-di-GMP levels through overproduction of GGDEF domain proteins led to enhanced adhesin and biofilm matrix component synthesis, while impairing motility and acute virulence functions. Conversely, overproduction of EAL domain proteins produced opposite phenotypes (Figure 1). Current research aims to assign specific molecular functions to GGDEF, EAL, and HD-GYP domain proteins and unravel their integration into complex regulatory networks. Notably, recent findings have identified biologically active GGDEF and EAL domain proteins lacking DGC or PDE activity, exemplifying their involvement in transcriptional regulation and RNA degradation.
 [5]
[5]
Molecular Interaction
The PilZ domain is typically found in proteins associated with type IV pili and other c-di-GMP-regulated processes. When c-di-GMP is present in the bacterial cell, it can bind to the PilZ domain, leading to conformational changes in the protein structure. This conformational change can result in alterations in protein-protein interactions or changes in protein activity, ultimately affecting the downstream processes regulated by the PilZ-containing protein. [6]
The binding of c-di-GMP to the PilZ domain is specific and occurs through conserved amino acid residues within the domain. The affinity and specificity of the interaction can vary among different PilZ-containing proteins, allowing for fine-tuning of c-di-GMP signaling in different bacterial species. [7]
Function in Virio cholera
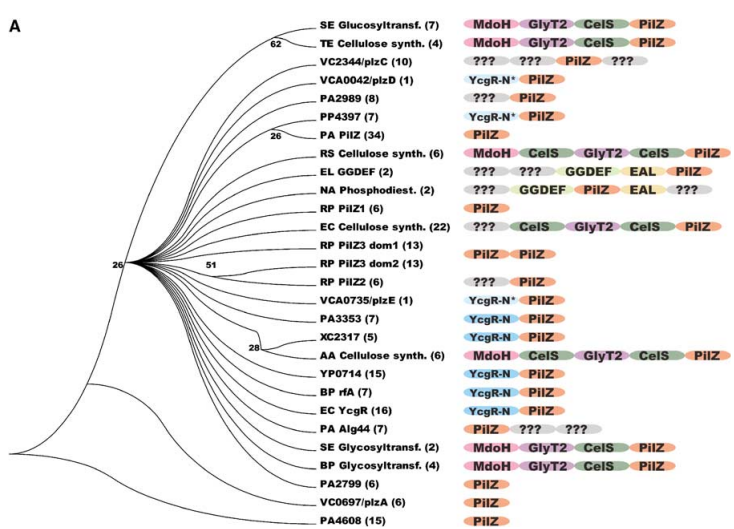
Cyclic diguanylate monophosphate (c-di-GMP) signaling plays a crucial role in the life cycle of Vibrio cholerae, a bacterium responsible for causing the human disease cholera. Cholera is characterized by potentially life-threatening diarrhea resulting from the secretion of cholera toxin. Studies have revealed that c-di-GMP regulates the expression of virulence genes in V. cholerae. Among the proteins involved in c-di-GMP signaling is VieA, a c-di-GMP phosphodiesterase that modulates cholera toxin production by influencing the cytosolic levels of c-di-GMP. Additionally, the genome of V. cholerae contains five PilZ domain-containing proteins, one of which is VCA0042. These proteins diverged in sequence before the divergence of the Vibrio lineage (Figure 1A) and are believed to function as regulators of various cellular processes controlled by c-di-GMP. VCA0042 has recently been named PlzD and has been shown to bind c-di-GMP when immobilized on nitrocellulose in its native conformation. Furthermore, a study has demonstrated that either VCA0042/PlzD or VC2344/PlzC, another PilZ domain-containing protein in V. cholerae, is required for efficient colonization of the intestines of mice by the El Tor biotype of V. cholerae in a virulence assay.
 [8]
[8]
Relevance Complements
Representation of c-di-GMP in 3D and 2D
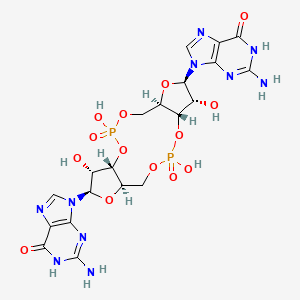
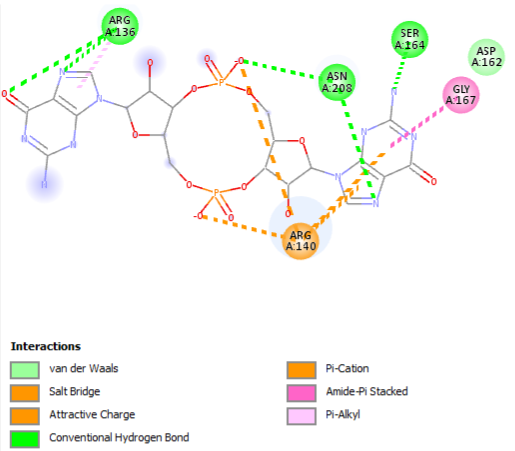
Representation of the interaction of the c-di-GMP (in yellow) with the most conservated residues from the active site
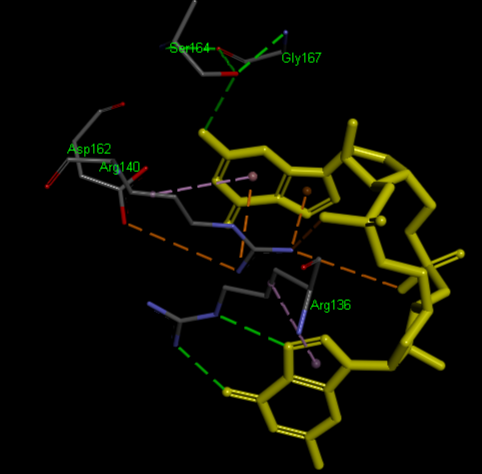
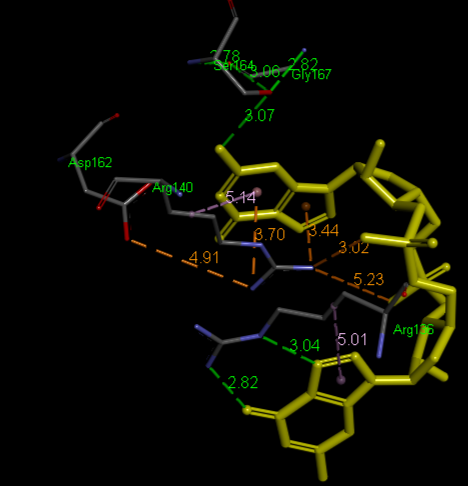
Structural highlights
Highlight representation of the the and of the protein, putting in evidence the alpha helix.
Crystal structure of VCA0042 complexed with c-di-GMP (2rde)
Pilz domain
(R136, R140, D162, S164, G167)
(R136, R140, D162, S164, G167)







