Chaperones are proteins that are involved in the non-covalent folding and unfolding of other macromolecules. It exists both in prokaryotes and eukaryotes. Some chaperones are constitutively expressed in the system however, there are other chaperones that are expressed only in response to an external stimulus or stress such as heat and therefore they are referred to as heat shock proteins.They are classified based on their structure, size, molecular weight and function in to several classes such as Hsp100, Hsp90, Hsp60 (chaperonin),small heat shock proteins (alpha)-crystallin proteins. They do not undergo denaturation themselves when exposed to stress because of better hydrogen bonding, strong hydrophobic core interactions, enhanced secondary structure and helix dipole stabilization.
Function
Chaperones bind to the newly synthesized proteins helping them acquire their properly folded 3D structure [1] Besides, chaperones help in targeting the native proteins to their respective organelles [2][3] The first identified chaperones were the histone chaperones that are continously involved in histone metabolism thus regulating genome function, stability and identity[4]. Many protozoan parasites such as Plasmodium falciparum requires these proteins for cytoprotection [5] [6]Chaperones actively participate in the maintenance of proteome integrity, and protein homeostasis (proteostasis) which requires a syncrhonization in various chaperones tuning the process [7].
Disease
Chaperones are instrumental in protein folding processes. Any alteration in this process leads to protein aggregation and formation of inclusion bodies. Protein misfolding results in various diseases such as Alzheimer[8],Cytosolic neurofibrillatory tangles,Parkinson[9],Familial amyotrophic lateral sclerosis, Huntington, Spinocerebellar ataxia 1, 2, 3, disease, Spinobulbar muscular atrophy and ageing[10].
Relevance
Modulation of chaperones expression is the new therapeutic approach for neurodegenerative and other disease arising from protein misfolding. There is a distinct network of chaperones and co chaperones that either directly influences the substrate proteins or in association with the protein degradation pathways such as the ubiquitin-proteasome-system or autophagy, results in the removal of completely misfolded and pathogenic proteins[11].
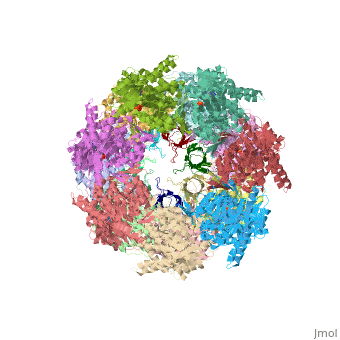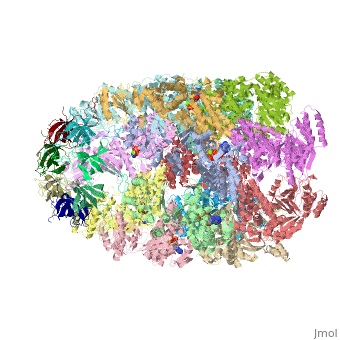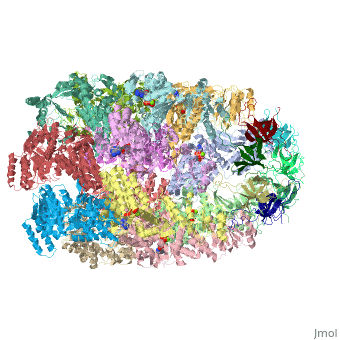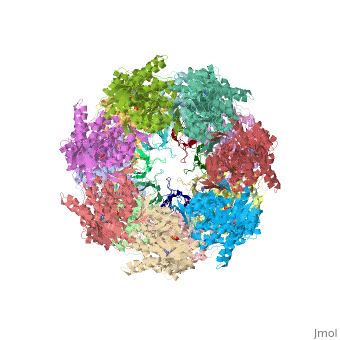
Structural highlights
Structurally, heat shock proteins have a N-terminal followed by a with elongated C-terminal. is a representative example of a chaperone system in complex with ADP.