This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Grace Mortimer/Sandbox 1
From Proteopedia
Contents |
Introduction and Function
Aquaporins are membrane proteins that function as water channels. They help the cells to regulate the volume and internal osmotic pressure of the cell using osmotic gradients. From a clinical viewpoint, aquaporins are crucial to maintaining homeostasis as well as exocrine fluid secretion and epidermal hydration [1]. Aquaporins are a part of a larger group of intrinsic proteins that form pores that span the plasma membranes of cells. Within mammals there are thirteen different types found, with ten being found in humans. Aquaporin 5 is a protein encoded by the AQP5 gene on chromosome 12, band 13. It is closely related to AQP0, AQP2, AQP5, and AQP6 [2]. Aquaporin-5 within humans osmotically facilitates the transport of water molecules across the plasma membrane while excluding any protons or ions, and it has been found in the cells of the stomach, duodenum, pancreas, airways, lungs, salivary glands, sweat glands, eyes, lacrimal glands, and the inner ear [3]. It is one of the only aquaporin proteins to be found in sweat glands and is found abundantly within the apical plasma membrane. Within sweat glands, Aquaporin-5 plays a crucial role as it is essential to humans’ ability to secrete sweat. Sweat itself is important to thermoregulation and understanding Aquaporin-5’s role in the secretion of sweat could be important in solving the problem of hyperhidrosis [4]. Aquaporin proteins can be split into two subgroups which are aquaporins and aquaglyceroporins, with Aquaporin-5 belonging to aquaporin subgroup, meaning that they are only permeable to water [5].
|
Structure
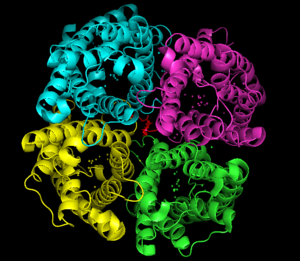
Aquaporin-5 is a homotetramer meaning that it contains four identical protomers and has a sequence length of 266. There protomers are made up of six transmembrane alpha helices as well as two half helices that essentially become a seventh transmembrane helix. The N and C-terminal are both located within the cytosol of the cell. Each of these transmembrane helices can be numbered one to six, with the two half helices being labeled B and E. These helices form loops with the half helix B set up between helices two and three, and the half helix E sitting between helices five and six [3]. The half helices begin in the middle of the membrane contain the (asparagine, proline, and alanine) near the center of the water channel. These two NPA motifs are hydrophobic and so they are the most important structural domains that play a crucial role in water-selective permeation in aquaporin water channels [6]. It is generally agreed upon that an electrostatic potential barrier that is strongest around this NPA motif also prevents protons from flowing through the channel. The water molecules move through the protomers themselves rather than through the channel that is formed by the monomers, since there is a that blocks water molecules, as well as any gases or ions, from flowing through. At the end of the monomer channels there is an (ar/R) constriction point which acts as an energy barrier and imposes substrate selectivity of the channel [7].
Phosphorylation of Aquaporin-5
Aquaporin-5 within humans has the ability to move from an intracellular compartment to the plasma membrane and back based on chemical signaling in the cell. This occurs due to phosphorylation of the protein which can occur at , as well as Thr259 and sites . Ser231 and Ser233 are located near the beginning of the C-terminal facing towards the cytoplasm which makes them easily accessible for phosphorylation. Ser152 and Ser156 also are found near the cytoplasm. The phosphorylation that occurs at Ser156 is done by protein kinase A. It is also important to note that the phosphorylation of Ser156 is the preferred phosphorylation point when it happens within cancerous cells. Several diseases are linked to the proteins ability to traffic itself, including Sjögren syndrome which can lead to dry eyes and mouth and caused by an inability of the protein to move from its intracellular compartment correctly [8].
Diseases and Mutations
Missense mutations in the AQP5 gene has been found to be linked to autosomal dominant nonepidermolytic palmoplantar keratoderma (PPK) which is essentially a thickening of the skin on the hands and feet. When studied, there were five APQ5 variants found in families that suffered from PPK. The first three variants are ones that affect the monomer channel’s restriction point while the other two variants are located on the extracellular surface of the protein. The first variant was a substitution of . With this mutation, it was substituted with a cysteine. This arginine is a part of the Ar/R constriction point which means that this substitution with a cysteine would result in the widening of the channel at the critical constriction point. The second variant is the substitution of on alpha helix two with a serine which would also result in the widening of the constriction point which would have a similar effect as the first variant. The third variant when the on alpha helix five is replaced with a phenylalanine would also have an effect on the size of the channel due to steric hinderance. The fourth and fifth variant have a similar effect to each other, as there substitutions, with Glu and with Asp, would affect the position of helix five due to the intramolecular reactions between these residues no longer happening. All of these variants would affect the protein’s ability to regulate the water that flows through them [7].
Mutations in Aquaporin-5 has also been linked to an overproduction of mucus and lowered lung function. A study done on smokers with COPD showed that there were three single nucleotide polymorphisms in the AQP5 gene that significantly affected the rate of lung function decline. This study showed overall that the APQ5 gene and the proper function of the Aquaporin-5 protein is important when looking into slowing the rate of lung function decline and the effects of COPD on a person.
Implications in Cancer
Overexpression of Aquaporin-5 has been linked to different cancers including colorectal cancers and small cell lung cancer.
It has been found that Aquaporin-5 is often overexpressed in colon cancer cell lines and tissues with a study showing that 62.8% of the tissue samples tested expressed the protein and 49.2% showed over expression. The same study showed that Aquaporin-5 causes cell proliferation in colon cancer cell lines. Some implications of this study shows that these same colon cancer cell lines growth can be inhibited if the Aquaporin-5 is targeted using siRNA [8].
Looking at small cell lung cancer, it was found that when the aquaporin-5 protein is present there was a higher rate of tumor recurrence and a lower chance of survival. It was found that when mutated versions of the protein were used in experiments, one that blocked the ability of the protein to move in the membrane and one that blocked the phosphorylation of Ser156, the normal protein induced cell invasions while the mutated ones did not [9].
References
- ↑ Verkman, A S. “Aquaporins in Clinical Medicine.” Annual Review of Medicine, U.S. National Library of Medicine, 1 Jan. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3319404/#:~:text=The%20aquaporins%20are%20a%20family,%2C%20epilepsy%2C%20and%20obesity).
- ↑ "AQP5 Aquaporin 5 [ Homo Sapiens (Human) ].” NCBI, 2 Mar. 2021, AQP5 aquaporin 5 [ Homo sapiens (human) ].
- ↑ 3.0 3.1 Horsefield, Rob, et al. “High-Resolution x-Ray Structure of Human Aquaporin 5.” PNAS, National Academy of Sciences, 9 Sept. 2008, www.pnas.org/content/105/36/13327#:~:text=Human%20aquaporin%205%20(HsAQP5)%20facilitates,glands%2C%20and%20the%20inner%20ear.
- ↑ Nejsum, Lene N., et al. “Functional Requirement of Aquaporin-5 in Plasma Membranes of Sweat Glands.” PNAS, National Academy of Sciences, 8 Jan. 2002, www.pnas.org/content/99/1/511.
- ↑ Blaydon, Diana C, et al. “Mutations in AQP5, Encoding a Water-Channel Protein, Cause Autosomal-Dominant Diffuse Nonepidermolytic Palmoplantar Keratoderma.” American Journal of Human Genetics, Elsevier, 8 Aug. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3738836/.
- ↑ Guan XG;Su WH;Yi F;Zhang D;Hao F;Zhang HG;Liu YJ;Feng XC;Ma TH; “NPA Motifs Play a Key Role in Plasma Membrane Targeting of Aquaporin-4.” IUBMB Life, U.S. National Library of Medicine, 2010, pubmed.ncbi.nlm.nih.gov/20186918/#:~:text=The%20two%20highly%20conserved%20NPA,AQP)%20biogenesis%20remain%20largely%20unknown.
- ↑ 7.0 7.1 Blaydon, Diana C, et al. “Mutations in AQP5, Encoding a Water-Channel Protein, Cause Autosomal-Dominant Diffuse Nonepidermolytic Palmoplantar Keratoderma.” American Journal of Human Genetics, Elsevier, 8 Aug. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3738836/.
- ↑ 8.0 8.1 Kang, Sung Koo, et al. “Role of Human Aquaporin 5 In Colorectal Carcinogenesis.” The American Journal of Pathology, Elsevier, 16 Dec. 2010, www.sciencedirect.com/science/article/pii/S0002944010616276.
- ↑ 5.Hansel, Nadia N., et al. “Aquaporin 5 Polymorphisms and Rate of Lung Function Decline in Chronic Obstructive Pulmonary Disease.” PLOS ONE, Public Library of Science, 3 Dec. 2010, journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0014226.