User:Henna Devi/melanocortin 1 receptor
From Proteopedia
|
Contents |
Melanocortin 1 Receptor: An insight to MCR1 structure, function and regulation
Introduction to MC1R
The highly polymorphic human MC1R gene, located on chromosome 16q24.3 encodes for a MC1R receptor. The MC1R gene is expressed mainly in melanocytes, which are melanin-producing skin cells, located on the plasma membrane along with other cell types that inhabit the skin such as keratinocytes and fibroblast as well as cells that operate the immune system (Gruis and Doorn, 2012). Herraiz et al., (2017) describes MC1R as ‘the most important regulator involved in all processes for producing pigments and the distribution of pigments around the skin’. It has a high affinity for the α-melanocortin stimulating hormone which aids in pigment production. MCR1 stimulation also results in increased melanocyte cell count, proliferation, cell survival and DNA repair. Herraiz et al., (2017) describes MC1R as ‘the most important regulator involved in all processes for producing pigments and the distribution of pigments around the skin’. It has a high affinity for the α-melanocortin stimulating hormone which aids in pigment production. MCR1 stimulation also results in increased melanocyte cell count, proliferation, cell survival and DNA repair.
Structure of the MC1R
The mature MC1R protein is made up of 317 amino acids and a 7 a-helical transmembrane domain. Based on the sequence similarity analysis, the melanocortin receptor family belong to the class A of G-coupled protein receptors therefore direct information on their secondary and tertiary structures is limited as G coupled protein receptors are resistant to crystallisation (Garcia-Borron, Sanchez-Laorden and Jimenez-Cervantes, 2005) (Zhao and Wu, 2012). Wolf Horrell, Boulanger and D’Orazio describe the melanocortin 1 receptor in four catergoies: The N and C terminus and the Intracellular and Extracellular loops. The N-terminus is the amino-terminal extracellular head which plays an important role in ligand affinity. The C terminus is the carboxyl-terminal cytoplasmic tail which is 14 amino acids long and is necessary for the trafficking of proteins affecting receptor localisation to the plasma membrane (Sharma and Schiller, 2019). As a short residue, the c-terminal uses molecular recognition for its function through its polarity. There are 3 intracellular loops and 3 extracellular loops, found between the transmembrane regions of MCR1. The Intracellular loops play an important role in G-coupled proteins for binding and signalling. MCR1 extracellular loops are essential for signalling activity. Extracellular loop 3 is crucial for cell surface receptor expression (Barwell, Conner and Poyner, 2011).
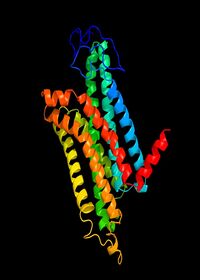
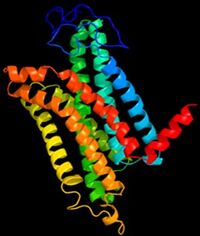
Visualising the 3D MC1R structure
As a GPCR there is a paucity of structural data (Dastmalchi, Church and Morris, 2008) as general difficulties that have been long known in crystalising membrane proteins, the flexibility of the receptor causes obstruction. The known difficulty in crystalising G-coupled proteins may be the significant reason for the incomplete 3D structural data for the melanocortin 1 receptor protein. However two structures with great similarlity have been detected for the MC1R , Figure 1. and Figure 2. are estimated 3D structures. The 7 helices are shown in different colours.
MC4R Ligands ,
, , ,
MC1R Signalling pathways: Pigmentation
Upon binding, as a G-coupled protein receptor, MCR1 regulates in multiple signalling pathways. MCR1 is activated by melanocortin agonists which are formed by keratinocytes, where pigments are moved to. When Gαs protein separates from MC1R it stimulates adenylyl cyclase which uses ATP to produce cAMP, a secondary messenger. The activation of the cAMP cascade stimulates multiple signalling pathways, in the production of melanin, an important component is protein kinase A (PKA). According to Grosse et al., (2008) PKA has two PKA subunits within its complex which are bound by two PKA regulatory subunits which allows binding to protein kinase C and cAMP. When the PKA regulatory subunits bind to two molecules of cAMP they separate and activate the subunit which can then bind to target proteins.Cyclic AMP is then produced to prevent activation of PKA. PKA stimulation increase the activity of tyrosinase.The activation of the cAMP cascade stimulates multiple signalling pathways, in the production of melanin, an important component is protein kinase A (PKA). According to Grosse et al., (2008) PKA has two PKA subunits within its complex which are bound by two PKA regulatory subunits which allows binding to protein kinase C and cAMP. When the PKA regulatory subunits bind to two molecules of cAMP they separate and activate the subunit which can then bind to target proteins. Cyclic AMP is then produced to prevent activation of PKA.
MC1R Signalling pathways: Anti-inflammatory properties
As well as its important role in pigmentation, the MC1R has anti-inflammatory properties for disease on macrophages.An alternative pathway of the MC1R which is more related to the role of MC1R in anti-inflammatory effects.Here the importance of protein kinase c produced by cAMP which leads to production of calcium and activates IP3. IP3 activates MAPK and JAK-STAT signalling pathways which result in the activation of CREB. CREB is a transcription factor which has anti-inflammatory properties by regulating the rise of anti-inflammatory genes (Zhang et al., 2020). This supports the argument that MC1R has anti-inflammatory properties.
Associated diseases
Melanoma
Melanoma is a type of skin cancer which is commonly recognised through the detection of a new mole or noticeable changes in a mole which was already developed (NHS Choices, 2019).Although melanoma can arise through multiple pathways it is most related to UV exposure often from the intense exposure of people with the phenotype of pale skin, red/blonde hair. These low penetrance MCR1 genes can interact with sun exposure leaving an individual at high risk of melanoma. Eumelanin is a photoprotective pigment therefore protects melanocytes from UV radiation however pheomelanin is phototoxic and generates oxidative stress (Hu et al., 2014). MC1R mutations are related to melanoma risk (Eggermont, Spatz and Robert, 2014) when they result in change in eumelanin production to pheomelanin. The more MC1R the more melanoma cells can migrate due to decreasing MAPK kinase which in turn increases syndecane-2 (Rosenkranz et al., 2013). The more MC1R is expressed the more cell division which leads to the overexpression of MC1R within the melanoma tumour.
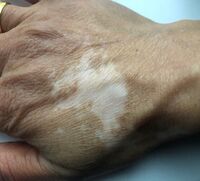
Vitilgo
Vitiligo is an autoimmune disease where the immune system attacks melanocytes in the skin resulting in a loss of pigmentation (medlineplus.gov, n.d.), an example of vitiligo is shown in figure 8 . The condition exists in two forms nonsegmental and segmental vitiligo. Recent studies (Ocampo‑Garza et al., 2020) concluded that genes involved in the melanocortin system related to the re-pigmentation progress in stable vitiligo patients, mainly MCR1 with a probability <0.05 showing results were significant. In this research, the aim is to include the possible relationship between MC1R and vitiligo into the MC1R proteopedia profile due to significant results shown in recent studies. Shen et al., (2016) explain that the Arg160Trp allele of MC1R gene can protect against vitiligo.
Drug binding: Afamelanotide
Afamelanotide is a potent drug which binds to the melanocortin-1 receptor and stimulates melanocyte proliferation and melanogenesis, therefore a MC1R agonist.By binding to the MC1R, afamelanotide causes the same downstream affects as the α- melanocyte stimulating hormone however its binding lasts longer. It is thought to be a possible treatment for vitiligo as it increases the production of eumelanin.
References
Barwell, J., Conner, A. and Poyner, D.R. (2011) Extracellular loops 1 and 3 and their associated transmembrane regions of the calcitonin receptor-like receptor are needed for CGRP receptor function. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research [online]. Preprint (10), pp.1906–1916. [Accessed 1 April 2021]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3228915/.
Dastmalchi, S., Church, W.B. and Morris, M.B. (2008). Modelling the structures of G protein-coupled receptors aided by three-dimensional validation. BMC Bioinformatics, [online]. 9(S1). . [Accessed 1 April 2021]. Available at:< Modelling the structures of G protein-coupled receptors aided by three-dimensional validation - PubMed (nih.gov)>
Eggermont, A.M., Spatz, A. and Robert, C. (2014). Cutaneous melanoma. The Lancet, [online] 383(9919), pp.816–827. [Accessed 3 March 2021]. Available at: https://www-sciencedirect-com.ezproxy.wlv.ac.uk/science/article/pii/S0140673613608028?via%3Dihub
Grosse, C., Heinekamp, T., Kniemeyer, O., Gehrke, A. and Brakhage, A.A. (2008). Protein Kinase A Regulates Growth, Sporulation, and Pigment Formation in Aspergillus fumigatus. Applied and Environmental Microbiology, [online] 74(15), pp.4923–4933.[ Accessed 3 March 2021]. Available at: < https://pubmed.ncbi.nlm.nih.gov/18539819/>.
Garcia-Borron, J.C., Sanchez-Laorden, B.L. and Jimenez-Cervantes, C. (2005). Melanocortin-1 receptor structure and functional regulation. Pigment Cell Research, [online] Preprint, p.051103015727002. [Accessed 3 March 2021]. Available at: https://pubmed.ncbi.nlm.nih.gov/16280005/.
Gruis, N.A. and Doorn, R. van (2012). Melanocortin 1 Receptor Function: Shifting Gears from Determining Skin and Nevus Phenotype to Fetal Growth. Journal of Investigative Dermatology, [online] 132(8), pp.1953–1955. [Accessed 8 March 2021]. Available at: <https://www.jidonline.org/article/S0022-202X(15)35846-2/fulltext>
Herraiz, C., Garcia-Borron, J.C., Jiménez-Cervantes, C. and Olivares, C. (2017). MC1R signaling. Intracellular partners and pathophysiological implications. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, [online] 1863(10), pp.2448–2461. [ Accessed 7 March 2021]. Available at: < https://www.sciencedirect.com/science/article/pii/S0925443917300765>.
Medlineplus.gov. (n.d.). Vitiligo: Medline Plus Genetics. [online]. [ Accessed 17 April 2021]. Available at:< https://medlineplus.gov/genetics/condition/vitiligo/>.
NHS Choices (2019). Overview - Skin cancer (melanoma). [online] [ Accessed 19 March 2021]. Available at:<.https://www.nhs.uk/conditions/melanoma-skin-cancer/>. Ocampo‑Garza, J., Salinas‑Santander, M., Welsh, O., Herz‑Ruelas, M. and Ocampo‑Candiani, J. (2020). Expression of melanocortin 1 receptor before and after narrowband UVB phototherapy treatment in patients with stable vitiligo: A prospective study. Experimental and Therapeutic Medicine. [online] Preprint. [ Accessed 2 March 2021]. Available at:< https://pubmed.ncbi.nlm.nih.gov/32104216/>
Rosenkranz, A.A., Slastnikova, T.A., Durymanov, M.O. and Sobolev, A.S. (2013). Malignant Melanoma and Melanocortin 1 Receptor. Biochemistry. Biokhimiia, [online] 78(11), pp.1228–1237. [ Accessed 15 April 2021]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4064721/#:~:text=Melanocortin%20receptor%2D1%20(MC1R).
Sharma, S. and Schiller, M.R. (2019). The carboxy-terminus, a key regulator of protein function. Critical reviews in biochemistry and molecular biology, [online] 54(2), pp.85–102. [ Accessed 15 April 2021]Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6568268/.
Shen, C., Gao, J., Sheng, Y., Dou, J., Zhou, F., Zheng, X., Ko, R., Tang, X., Zhu, C., Yin, X., Sun, L., Cui, Y. and Zhang, X. (2016). Genetic Susceptibility to Vitiligo: GWAS Approaches for Identifying Vitiligo Susceptibility Genes and Loci. Frontiers in Genetics [online] , 7. [ Accessed 15 April 2021]Available at: < https://www.frontiersin.org/articles/10.3389/fgene.2016.00003/full>.
Wolf Horrell, E.M., Boulanger, M.C. and D’Orazio, J.A. (2016). Melanocortin 1 Receptor: Structure, Function, and Regulation. Frontiers in Genetics, [online] 7 preprint [ Accessed 15 April 2021] Available at:< https://www.frontiersin.org/articles/10.3389/fgene.2016.00095/full>.
Zhang, C., Chery, S., Lazerson, A., Altman, N.H., Jackson, R., Holt, G., Campos, M., Schally, A. V and Mirsaeidi, M. (2020). Anti-inflammatory effects of α-MSH through p-CREB expression in sarcoidosis like granuloma model. Scientific Reports, [online] 10 [Accessed 20 April 2021] . Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7190699/ .
Zhao, Q. and Wu, B. (2012). Ice breaking in GPCR structural biology. Acta Pharmacologica Sinica [online] , 33(3), pp.324–334[ Accessed 15 April 2021] Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4077136/.