User:James Lignos/Sandbox 1
From Proteopedia
Contents |
Bromodomain of PfBDP1
|
Function
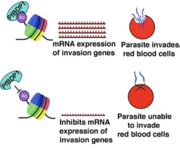
The bromodomain in PfBDP1, a protein in the malaria parasite Plasmodium falciparum, functions as a reader of histone acetylation, specifically binding to acetylated lysine residues on histone tails. This interaction allows PfBDP1 to regulate gene expression, particularly of genes involved in erythrocyte invasion. PfBDP1's bromodomain helps tether a transcriptional activator complex to acetylated histones, influencing the expression of genes required for parasite growth and invasion
Disease
Malaria pathogenesis involves several stages, primarily the asexual and sexual cycles of the Plasmodium parasite within the human host and the mosquito, respectively. The parasite invades liver cells (exoerythrocytic stage) and subsequently infects red blood cells (erythrocytic stage), causing symptoms like fever, chills, and anemia. The symptoms of malaria are associated with repeated rounds of parasite replication, egress, and invasion into the red blood cells. At the red blood cell stage of infection, P. falciparum consumes the RBCs hemoglobin, preventing it from carrying oxygen to the heart, which results in anemic heart failure4. In addition, parasitized RBCs stick to the wall of blood vessels in the heart and brain to evade the immune system, which often leads to inflammation and causes blood vessel blockage in these vital organs. These infection-related complications are directly associated with the invasion of RBCs by the parasite
Relevance
The most recent data from the World Health Organization (WHO) indicates that in 2023, there were an estimated 263 million malaria cases and 597,000 deaths globally. Treatment options are very limited and drug resistance is a major hurdle in combating malaria. The prevalence of Malaria coupled with the limited treatment options underpins the need for advancements in therapeutic strategies
References
References Gilan, O., Rioja, I., Knezevic, K., Bell, M. J., Yeung, M. M., Harker, N. R., Lam, E. Y. N., Chung, C. W., Bamborough, P., Petretich, M., Urh, M., Atkinson, S. J., Bassil, A. K., Roberts, E. J., Vassiliadis, D., Burr, M. L., Preston, A. G. S., Wellaway, C., Werner, T.,…Dawson, M. A. (2020). Selective targeting of BD1 and BD2 of the BET proteins in cancer and immunoinflammation. Science, 368(6489), 387-394. https://doi.org/10.1126/science.aaz8455 Gokani, S., & Bhatt, L. K. (2021). Bromodomains: A novel target for the anticancer therapy. Eur J Pharmacol, 911, 174523. https://doi.org/10.1016/j.ejphar.2021.174523 Josling, G. A., Petter, M., Oehring, S. C., Gupta, A. P., Dietz, O., Wilson, D. W., Schubert, T., Langst, G., Gilson, P. R., Crabb, B. S., Moes, S., Jenoe, P., Lim, S. W., Brown, G. V., Bozdech, Z., Voss, T. S., & Duffy, M. F. (2015). A Plasmodium Falciparum Bromodomain Protein Regulates Invasion Gene Expression. Cell Host Microbe, 17(6), 741-751. https://doi.org/10.1016/j.chom.2015.05.009 Miller, L. H., Good, M. F., & Milon, G. (1994). Malaria pathogenesis. Science, 264(5167), 1878-1883. https://doi.org/10.1126/science.8009217 Singh, A. K., Phillips, M., Alkrimi, S., Tonelli, M., Boyson, S. P., Malone, K. L., Nix, J. C., & Glass, K. C. (2022). Structural insights into acetylated histone ligand recognition by the BDP1 bromodomain of Plasmodium falciparum. Int J Biol Macromol, 223(Pt A), 316-326. https://doi.org/10.1016/j.ijbiomac.2022.10.247 (Gilan et al., 2020; Gokani & Bhatt, 2021; Josling et al., 2015; Singh et al., 2022)