User:Kai D. Ludwig/Sandbox 1
From Proteopedia
Contents |
PYRUVATE KINASE
INTRODUCTION
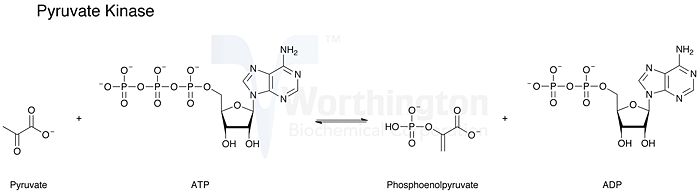
Pyruvate Kinase (PK) is the enzyme in the final step of aerobic glycolysis. The enzyme catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to an adenosine diphosphate (ADP) to make both pyruvate and adenosine triphosphate (ATP)[1]. Figure 1 illustrates the chemical reaction catalyzed by pyruvate kinase. The purpose in performing glycolysis is for a cell to create energy in the form of ATP as well as to make pyruvate. Pyruvate can be then converted and used to create even more energy in the cell. Pyruvate can either be directed to enter the Krebs cycle when in aerobic (oxygen present) conditions or be converted to lactate in anaerobic glycolysis (oxygen not present). This reaction has a large negative free energy or release of energy that results from the substrate level phosphorylation (making of ATP). The negative or Gibbs free energy that results from breaking a phosphate group off of phosphoenolpyruvate is 12.3 kcal/mol or 51.6 kJ/mol.[2] Being such a significantly large release of energy, the reaction is essentially irreversible. This allows the process to be a regulator in the glycolytic process. Amounts of reactants and products can be controlled because in this reaction, products are almost always formed by PK when active. If the cell needs more of the reactants, PK can be inactivated. Pyruvate Kinase is regulated by its own substrates.[3] Increased regulation occurs when PEP is high in concentration and contrarily, PK is inhibited in the presence of its by-products: ATP and pyruvate. It is intuitive that pyruvate kinase would increase its catabolic activities in the presence of more PEP so it can make more energy (ATP) and create pyruvate. Unless a cell already contains large amount of ATP and pyruvate, hence they inhibit pyruvate kinase.
ISOZYMES
The human has several different forms of pyruvate kinase. The two different isozymes are the L/R and M form. They are isozymes because the two forms differ greatly in there amino acid sequence but have the same function. The isozymes are tissue specific and are named accordingly: L (liver), R (erythrocyte), and M (Muscle). The muscle form can be either considered M1 or M2. M1 is found primarily in muscle, heart and brain and the M2 form is found in fast proliferating cells such as early fetal tissue and cancers as well as in the kidney.[4] The canonical form or primary form considered in literature is the R-type of pyruvate kinase. The R-type found in red blood cells is very similar to the L-type found in hepatocytes and differ only in the sequence of the first 33 amino acids. The sequence differs as follows: MSIQENISSLQLRSWVSKSQRDLAKSILIGAPG (R)→ ME (L).[5,6] This sequence difference may lead to a difference in allosteric regulation properties. The M1 and M2 form have a major difference in their amino acid sequence from the R/L form. Yet, they have the same catalyitic activity. The M1 differs from the M2 type in its sequence from amino acids 389-433 as is as follows: IYHLQ LFEEL RRLAP ITSDP TEATA VGAVE ASFKC CSGAI IVLTK (M2)→ MFHRK LFEEL VRASS HSTDL MEAMA MGSVE ASYKC LAAAL IVLTE (M1).[7,8] This sequence difference leads to binding activities and regulation of its activity that are discussed later.
STRUCTURE
|
Pyruvate Kinase in its active form is a homotetramer. This means it consists of four identical subunits or monomers. The can be seen in different colors. Each also consists of four different domains. They are the N-terminal, A Domain, B Domain, and C-terminal.[9] The primary structure of the R-type pyruvate kinase is 574 amino acids long (531 amino acids for PKM2) which arrange themselves into secondary structures for stability. Pyruvate kinase contains both alpha helixes as well as beta sheets. The can be seen as alpha helixes in blue and beta sheets in red. Each subunit of pyruvate kinase contains all four domains as well as an active site. These different “pieces” of pyruvate kinase do not facilitate a reaction as individual parts very well. Instead, they come together to form the homotetramer or quaternary structure, which increases the enzymes ability to catalyze the reaction. In addition to needing all four subunits, cofactors are necessary to function. Each monomer requires specific metals: Mg2+ (or ) and . These cofactors interact with specific amino acids. Potassium binds to four amino acid residues: Asparagine75, Serine77, Aspartate113, and Threonine114. Magnesium binds to Glutamic Acid272 and Aspartic Acid296. This allows for the enzyme to change conformation or overall shape. When bound, pyruvate kinase changes from its low affinity T state to its high substrate affinity R state. This can be seen in figure 2. The figure illustrates the more inactive T-state on left and the more active state R-state on the right which has a more donut shape. The active sites can be noted in yellow with the metal binding sites in green. The magenta binding sites are for other allosteric binding molecules such as FBP. This action can be described as the enzyme ‘breathing’ as it contracts and expands in the presence of co-factors. Other co-factors include and . Both PGA and FBP are glucose derivatives found upstream (earlier) in glycolysis from pyruvate and PEP. FBP allosterically activates pyruvate kinase.[11] In the absence of FBP, pyruvate kinase exists as a monomer. However when FBP is around, the sugar molecule binds PK and promotes the homotetramer form to exist and increases its enzymatic properties. FBP binds at regions 432-437 and 514-521. The catalysis site consists of six amino acids that bind and initiate the phospho-transfer from PEP to ADP. The can be seen here. The six amino acids are Arginine, Arginine, Lysine, Threonine, Serine, and Glutamate.[9] Together these six amino acids allow the two reactants to enter into the active site, stabilize the intermediates, and release the products.

TUMOR PKM2
Tumor cells undergo a change in their metabolism of glucose that is described as the Warburg effect.[14] Glucose is broken down into pyruvate and instead of being directed into oxidative phosphorylation, pyruvate is converted into lactate to make ATP, even in the presence of oxygen. Pyruvate kinase seems to play a role in tumorigenesis. The M2 form of pyruvate kinase has been identified in fetal tissue and other fast proliferating cells. This includes cancers. PKM2 has been found in many types of tumors and as these tumors grow the expression of other forms of pyruvate kinase disappear as PKM2 becomes highly expressed.[10] I have already mentioned, the M2 form differs from the M1 in its sequence in the region of amino acids 389-433. There are a total of 22 differences in sequence and they play a significant role in how the subunits of pyruvate kinase interact. This region of amino acids is found on each subunit on the ‘Y’ interface of the homotetramer protein. The interface is the plane in which two subunits are in close contact. The ‘Y’ interface is defined where the two upper subunits interact and another where the two lower subunits interact. The interactions between the subunits on the interface are weaker in the M2 form than the M1 form.[9] As a result, the M2 isoform also has a dimeric form that exists. The dimeric form plays a major role in pyruvate kinases activity and tumor metabolism.[13] This M2 sequence effectively allows the subunits to exist in a tetramer or dimeric form. The tetrameric form is the more active form and has a high affinity for its substrate PEP. The dimeric form on the other hand, has a low affinity for its substrate. This low affinity and less active state allows for the cancerous cell to channel its glucose carbons toward synthetic processes. The regulation of the quarternary structure of PKM2 is done by the glycolytic intermediates such as FBP.[11] Cancerous cells that express high amounts of PKM2 have a selective advantage in growth. A switch in the expression of PKM1 to PKM2 allows cancerous cells to maintain their high proliferating processes such as rapid DNA replication, protein synthesis, etc. Figure 6 shows a two proposed pathways for glucose metabolism in normal proliferating cells and tumor cells. A study has been done where the importance of switching the expression of pyruvate kinase M1 to M2 isoform was noted. By using short hairpin RNA, the expression of PKM2 was knockdown and was replaced by the M1 form in tumor cells. These cells then had lower lactate production, greater oxygen consumption, and essentially a reversal of the Warburg effect.[12]
ROLE OF PYRUVATE KINASE M2 IN TRANSCRPTION
Besides being a glycolytic enzyme, PKM2 has been shown to regulate transcription. PKM2 seems to regulate the transcription of the Oct-4 gene.[15] The Oct-4 gene encodes a transcription factor (Oct-4) that plays a role in stem cells and the maintaining of their pluripotent state.[16] It prevents the expression of genes that lead to stem cells losing this pluripotent state and differentiate. The C-Terminus on pyruvate kinase (amino acids 307-531) binds to Oct-4. By increasing concentrations of PKM2, expression of Oct-4 can be enhanced. PKM2 seems to up-regulate the expression of Oct-4. Oct-4 is found in stem cells and early fetal cells. In addition it has been found in many breast carcinomas and not in healthy, normal breast cells.[17] The mechanism by which cells over-expressing PKM2 and Oct-4 may help induce these cells to become cancerous.
NOTES AND REFERENCES
[1] Boyer, P.D. Pyruvate kinase. In: Boyer, P.D., Lardy, H. and Myrbäck, K. (Eds.) The Enzymes, 2nd ed. vol. 6, Academic [ress, New York, 1962, 95-113.
[2] Barton, Larry L.; Structural and Functional Relationships in Prokaryotes. Springer Science + Business Media. Inc. New York, 2005, pp318.
[3] Dann, Leighton G. and Britton, Hubert G.; Kinetics and Mechanism of Action of Muscle Pyruvate Kinase. Journal of Biochemistry. 1978 169:39-54.
[4] Consler, T.G.; Woodard, S.H.; and Lee, J.C. (1989). Effects of Primary Sequence Differences on the Global Structure and Function of an Enzyme; A study of pyruvate kinase isozymes. Biochemistry 28:8756-8764.
[5] Tani K., Fujii H., Nagata S., Miwa S.; Human liver type pyruvate kinase: complete amino acid sequence and the expression in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 85:1792-1795(1988)
[6] Giovanna Valentini, Laurent R. Chiarelli, Riccardo Fortin, Manuela Dolzan, Alessandro Galizzi, Donald J. Abraham, Changqing Wang, Paola Bianchi, Alberto Zanella, and Andrea Mattevi; Structure and Function of Human Erythrocyte Pyruvate Kinase”; The Journal of Biological Chemistry 277, 23807-23814. June 28, 2002
[7] Takenaka M., Noguchi T., Sadahiro S., Hirai H., Yamada K., Matsuda T., Imai E., Tanaka T.; Isolation and characterization of the human pyruvate kinase M gene. Eur. J. Biochem. 198:101-106(1991) [PubMed: 2040271]
[8] Dombrauckas J.D., Santarsiero B.D., Mesecar A.D.; Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44:9417-9429(2005)
[9] John O. Wooll, Robert H. E. Friesen, Mark A. White, Stanley J. Watowich, Robert O. Fox, J. Ching Lee and Edmund W. Czerwinski. Structural and Functional Linkages Between Subunit Interfaces in Mammalian Pyruvate Kinase. J. Mol. Biol. (2001) 312, 525±540
[10] Melissa S Jurica, Andrew Mesecar, Patrick J Heath, Wuxian Shi, Thomas Nowak, and Barry L Stoddard. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure, Volume 6, Issue 2, 195-210, 15 February 1998
[11] Dombrauckas J.D., Santarsiero B.D., Mesecar A.D.; Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry 44:9417-9429(2005)
[12] Heather R. Christofk, Matthew G. Vander Heiden , Marian H. Harris, Arvind Ramanathan, Robert E. Gerszten, Ru Wei, Mark D. Fleming, Stuart L. Schreiber & Lewis C. Cantley; The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230-233 (13 March 2008)
[13] Mazurek S; Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic prolfiling of tumors. 2007. Emst. Schering Found. Symp. Proc. 4: 99-124.
[14] Warburg O (1956). "On the origin of cancer cells". Science 123 (3191): 309–14. doi:10.1126/science.123.3191.309. PMID 13298683. 94250672
[15] Lee, J.; Kim, H.K.; Han, Y.M.; Kim, J. (2008). Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int. J. Biochem. and Cell Biol. 40:1043-1054.
[16] Pesce, M., & Scholer, H. R. (2001). Oct-4: Gatekeeper in the beginnings of mammalian development. Stem Cells, 19,271–278.
[17] Jin, T., Branch, D. R., Zhang, X., Qi, S., Youngson, B., & Goss, P. E. (1999). Examination of POU homeobox gene expression in human breast cancer cells. Int. J. Cancer, 81, 104–112.

![Figure 3:Figure 6: A metabolic strategy for tumor cells using PKM2 Taken from:[12]](/wiki/images/thumb/2/22/M2-pkcancer.jpg/300px-M2-pkcancer.jpg)
