This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Karen Lee/Sandbox 1
From Proteopedia
Replication is an essential process in all cells. The process copies the chromosomal DNA of the organism to provide the extra copy needed in cell division and is therefore critical in the biological inheritance of genes. In cells with circular chromosomes, replication starts from a single origin and proceeds with two replication forks moving in opposite directions. This process must be terminated, otherwise multiple copies of the chromosome would be made.
The process of terminating replication is performed by replication termination proteins. These proteins bind to specific sequences in the DNA, called Ter sites. This binding provides a physical blockage in the DNA that stops the replication machinery. In B. subtilis, the termination protein is called Replication Terminator Protein (RTP), and in E. coli it is Termination Utilisation Substance (Tus).
Each circular chromosome has two sets of Ter sites that appear roughly opposite to the origin of replication. One set blocks the clockwise replication fork while the other traps the anti-clockwise replication fork. Interestingly, both these proteins bind to DNA in a polar manner that blocks the replication fork in one direction, but allows it to continue when approached from the other direction.
Contents |
RTP
|
Structure of RTP
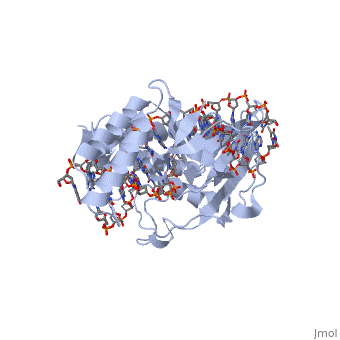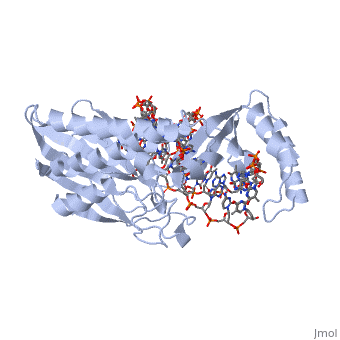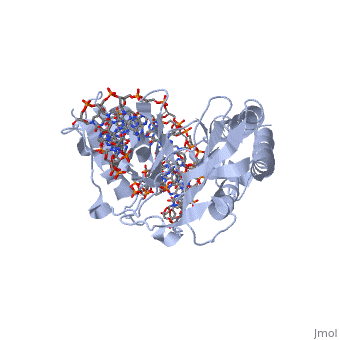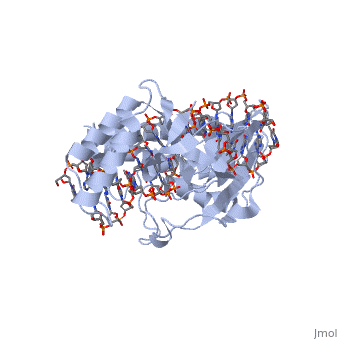
A single RTP monomer consists of four , three and an unstructured domain. The binds to DNA by into the major groove of Ter sites, while the interacts with the minor groove. The N-terminal arm also binds to the Ter site[1].
RTP conforms to the classic winged-helix motif, a compact α/β structure with αβααββ topology where "" project from the loop between and [2]. However, each monomer lacks a well-formed β1-loop and has an additional following the β3-strand[3].
The functional RTP-DNA complex requires two dimers of RTP. Dimerisation occurs through the extended of each monomer forming an anti-parallel coiled coil. The RTP dimer binds to the B site first which then facilitates cooperative binding of another RTP dimer to the A site to form a complete RTP-Ter complex[3]. In particular, the of the α4-helix associates with the complimentary region on the partner monomer[4].
This α4-helix has other structurally important features. Within the proximal third, residues contribute to the hydrophobic core of the RTP monomer. Additonally, (which is from the N-terminal strand) contribute to the hydrophobic core of the partner RTP monomer, acting as dimerisation elements[4].
The hydrophobic core also contains an aromatic network consisting of from the partner monomer. The interactions between these side chains represent an efficient method of providing protein stability[4].
It is thought that upon binding to the Ter sequence, the complex is able to impede the progress of replicative machinery headed by the replicative helicase. This is supported by in vitro studies involving E. coli where RTP was shown to interact with the DnaB helicase, thus a similar interaction may also occur in B. subtilis [5]. The RTP-helicase interaction has been identified localised to the anti-parallel 3-strand β-sheet surface. Residues form the hydrophobic patch, while residues show homology with the DnaB initiator protein[4].
()
Polarity
All of the Ter sites in the B. subtilis chromosome are only partially symmetric about the mid-point. However, initial studies of the RTP-DNA structure were performed using a symmetrically designed sequence (sRB) based on the TerI (the first Ter site that the clockwise replication fork encounters) B site [6]. NMR and crystallography revealed that RTP bound symmetrically to this symmetric sRB sequence. However, further studies using the non-symmetric native TerI B site (nRB) showed an asymmetric structure. Between the sRB and nRB sites there were only 6 base pair differences: three upstream and three downstream, but these changes were significant enough to influence the binding of RTP to DNA. Despite the change in sequence, the RTP-sRB interaction showed similar binding affinity to the RTP-nRB interaction, but had reduced fork arrest capability[6].
The three downstream base pair changes have no bearing on the conformation of RTP as no base-specific interactions are made in this region. However, the three upstream changes are located within the major groove of the dsDNA of the TerI site, where the binds. Thus, it is these 3 bases that underlies the RTP binding specificity[3].
The polar nature of the RTP-DNA complex at the complete Ter site results from the different binding affinites of RTP to the A and B sites. When the fork approaches the B site where there is tight RTP-DNA binding, the fork is unable to progress. But approaching from the A site, where there is a lower binding affinity, the fork is able to pass the complex. This is the basis of the polar mechanism of the RTP-DNA complex that traps the replication fork for termination.
Tus
|
Structure of Tus
The Tus protein functions as a monomer, each monomer consists of two domains, the and domains. The overall structure of Tus is made up of two separated by central twisted anti-parallel . These structures together form a that accommodates a B-form Ter . This binding involves two the bases and sugar-phosphate back bones in the major groove of the Ter DNA[7].
()
Polar fork arrest mechanism
Like the RTP Ter sites, the Tus Ter sites do not display any symmetry or inverted repeats, thus a polar complex is formed between the Tus protein and Ter site. Despite both being replication termination proteins, RTP and Tus have very different structures and mechanisms of polar fork arrest.
The polar binding of Tus to its Ter site results in the protein having 2 different faces: a non-permissive face where the replication fork is blocked to terminate replication, and a permissive face where the replication machinery, headed by the DnaB helicase is allowed to pass and continue replication.
DnaB helicase normally functions by clamping onto only one of the single stranded DNA stands of the replication fork, and sliding in a 5’→3’ direction to progressively unwind the double stranded DNA ahead. It is this sliding movement of the DnaB helicase along the DNA that knocks Tus off its Ter binding site when approached from the permissive face of Tus that allows replication to continue. Tus dissociation occurs due to the progressive loss of Tus-Ter interactions.
On the other hand, when the DnaB helicase approaches from the non-permissive face, the DnaB helicase is unable to knock Tus off its Ter site due to the formation of a locked complex between Tus and it’s duplex Ter sequence[8].
Of particular importance to the formation of this complex is the bottom strand cytosine at position 6 of the Ter site (C6), which has been strictly conserved in all ten E. coli Ter sites. Formation begins with the DnaB helicase separating the Ter site DNA from the non-permissive face to release C6 from its base pair partner. This then leaves C6 to be freely base flipped out of the double helix, through rotation of the phosphodiester backbone, and into a pocket near the α4 helix of the Tus protein. Hydrogen bonding with positions the within the pocket. C6 is not the only base that undergoes change, the adenine at position 7 (A7) of the bottom strand is also moved out of the helix to allow C6 to reach to the pocket. A7 does not seem to make any base specific contacts with the Tus protein, as supported by the lack of conservation at this position of the Ter site.
The unlocking of the complex after it has performed it function of terminating replication from the non-permissive face, is ultimately required for the replication fork in the other direction to proceed. There is no specific unlocking mechanism, this is simply achieved as previously described, with the sliding movement of the DnaB helicase knocking Tus off its Ter sequence.
References
- ↑ Pai, S. K., Bussiere, D. E., Wang, F., Hutchinson, C. A., White, S. W. & Bastia, D. (1996) The structure and function of the replication terminator protein of Bacillus subtilis: identification of the ‘winged helix’ DNA-binding domain. EMBO J. 15(12), 3164-3173.
- ↑ Gajiwala, K. S. & Burley, S. K. (2000) Winged Helix proteins. Curr. Opin. Struct. Biol. 10, 110-116.
- ↑ 3.0 3.1 3.2 Vivian, J. P., Porter, C. J., Wilce, J. A. & Wilce, M. C. J. (2007) An Asymmetric Structure of the Bacillus subtilis Replication Terminator Protein in Complex with DNA. J. Mol. Biol. 370, 481-491.
- ↑ 4.0 4.1 4.2 4.3 Bussiere, D. E., Bastia, D. & White, S. W. (1995) Crystal Structure of the Replication Terminator Protein from B. subtilis at 2.6Å. Cell. 80, 651-660.
- ↑ Gautam, A., Mulugu, S., Alexander, K. & Bastia, D. (2001) A Single Domain of the Replication Termination Protein of Bacillus subtilis Is Involved in Arresting Both DnaB Helicase and RNA Polymerase. J. Biol. Chem. 276(26), 23471-23479.
- ↑ 6.0 6.1 Wilce, J. A., Vivian, J. P., Hastings, A. F., Otting, G., Folmer, R. H., Duggin, I. G. et al. (2001) Structure of the RTP-DNA complex and the mechanism of polar replication fork arrest. Nature Struct. Biol. 8, 206-210.
- ↑ Kamada, K., Horiuchi, T., Ohsumi, K., Shimamoto, N. & Morikawa, K. (1996) Structure of a replication-terminator protein complexed with DNA. Nature. 383, 598-603.
- ↑ Mulcair, M. D., Schaeffer, P. M., Oakley, A. J., Cross, H. F., Neylon, C., Hill, T. M. & Dixon, N. E. (2006) A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. coli. Cell. 125, 1309-1319.
Page Contributors
Matthew Hoe, Karen Lee