User:Kristína Galvánková/Sandbox 1
From Proteopedia
|
ß-catenin is an intracellular adaptor protein, which can be a part of many protein complexes that respond to different biological signals. Depending on its interacting partners, ß-catenin elicits specific cellular response. This response serves to maintain the tissue homeostasis. Thus, it is not surprising that mutations affecting the structure and function of ß-catenin can lead to tumor transformation [1], defects in embryogenesis [2] and regeneration [3].
Contents |
Structure
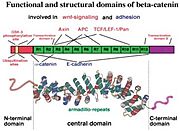
Human ß-catenin is a product of CTNNB1 gene and contains 781 amino acids. The main part of this protein displays α-helical conformation and includes 12 . Therefore, ß-catenin is classified as armadillo family member. Each armadillo repetition contains 42 amino acids folded into 3 α-helices, that are connected with short unstructured loops. The α-helices of neighboring armadillo repetitions interact and thus participate in the formation of a compact right-handed superhelical conformation. The inner region of ß-catenin protein that adopts this conformation and includes all 12 armadillo repetition is called ß59 (according to its molecular weight in kDa). Similar organization can be found also in other armadillo proteins, that use it as an interacting domain. The ß59 region has hydrophobic amino acids distributed at its core, whereas positively charged amino acids residues are exposed on its surface [4]. In this way, armadillo protein ß-catenin can interact with proteins of Cadherin-Catenin complex, Wnt signaling pathway [5], or with members of the transcription factor family [6].
Function
|
ß-catenin becomes a member of mechanosensory complex forming adherent junction between cells by interaction with transmembrane protein Cadherin. In this complex, ß-catenin serves as one of the mechanosensors, that transmit the traction force generated extracellularly into the cell. Thanks to that, cells are anchored to each other by their proteins and Actin cytoskeleton and can receive or transmit information about changes in surrounding tissue [7].
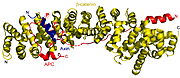
Interaction site for Cadherin on ß-catenin overlaps in part with interaction site for another protein – Fascin. Cadherin and Fascin compete each other for interaction with ß-catenin, where Cadherin interaction leads to adherent phenotype, whereas Fascin interaction results in motile cell. Another competitor of Fascin for attachment to ß-catenin is Adenomatous polyposis coli (APC) protein [9]. APC with Axin, Glycogen synthase kinase 3 (GSK3) and Casein kinase 1 (CK1) assemble a complex, that plays a role in Wnt signal pathway.
This complex participates in destabilization of soluble cytoplasmic ß-catenin and prevent interaction between ß-catenin and transcription factors.For example, ß-catenin with Tcf/Lef acts as co-activator of transcription, so that all these proteins must be together transported to the nucleus where they help to initiate pro-proliferative genes expression [10]. About participation of ß-catenin in these complexes decides mainly its tyrosine and serine/threonine phosphorylation [11] [12].
Mutations
Many naturally occurring mutations in the CTNNB1 gene have been associated with various pathologies such as pilomatrixoma [14], medulloblastoma [15], ovarian cancer [16] and neurodevelopmental disorder with spastic diplegia and visual defects (NEDSDV) [17]. However, defects in ß-catenin functions are mostly associated with the development of colorectal carcinoma [1]. Sequencing of CTNNB1 gene in 23 cancer cell lines from 21 patients with colorectal carcinoma revealed five mutant variants. Only one of these mutations has homozygous character [18].

Mutations discovered in cell lines SW48 and HCT116 target to positions 33 and 45 in exon 3 and affect the part of ß-catenin outside of armadillo repetitions. The mutation in SV48 cell line substitutes serine 33 by tyrosine. In HCT116 cell line, the deletion of three nucleotides eliminates serine in position 45. Both these changes in the primary structure of ß-catenin have negative effect on the binding site for GSK3 kinase (see Fig. 2), although in the case of serine 45 elimination in HCT116, the effect is only indirect. Enzyme GSK3 is processive serine/threonine kinase, that gradually phosphorylates several serine residues of ß-catenin. The priming phosphorylation of serine 45 by CK1 kinase is necessary for the interaction of GSK3 and ß-catenin [18].
The remaining three mutations interrupt the armadillo repetitions . In HCA46 cell line, the mutation hits that part of exon 5, which corresponds to the second armadillo repetition. New potential phosphorylation site here substitutes alanine by serine,. In Caco2 cell line, there is substitution of glycine to alanine, but this time in that part of exon 5, that corresponds to the third armadillo repetition. Both these mutations in both cell lines change the polarity and hydrophobicity in ß59 region, which in turn affects phosphorylation of ß-catenin and its interactions [18].
The last two cancer cell lines, Colo201 and Colo205, were derived from the same patient and represent the primary tumor and its metastases. In both cell lines, the same mutation in exon 6 in the fourth armadillo repetition was found. This mutation substitutes asparagine to serine, which affects the interactions of ß-catenin in complexes with APC and Cadherin [18].
Disease
Colorectal carcinoma is the third most common cancer worldwide and contributes substantially to the tumor-caused mortality. This type of cancer is the second most lethal cancer disease [19] and is specifically overrepresented in the developed parts of the world [20]. About 50% of patients with colorectal carcinoma develop metastases, which significantly diminishes the chance to survive [21].
In the case of colorectal cancer development, Wnt signal pathway is very important and represents the central point of carcinogenesis. As a result, activating mutations in this pathway are present in more than 80% of patients. Basically, Wnt pathway is activated, when an extracellular ligand (glycoprotein) binds to the receptor protein Frizzled, that is localized in the cytoplasmic membrane. Then, protein Frizzled can interact with its co-receptor LPR, which allows subsequent phosphorylation of cytoplasmic protein Dishevelled. When phosphorylated, this protein serves as an inhibitor of GSK3. Inactivation of GSK3 kinase prevents degradation of ß-catenin, that accumulates in cytoplasm and translocates to the nucleus, where it plays a role as transcription co-activator. Every step of this pathway is strictly regulated. After signal termination, cytoplasmic ß-catenin is degraded [22].
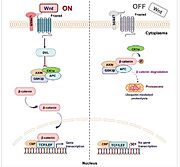
Protein APC is very important for this pathway and for the decreasing ß-catenin stability. Therefore, the majority of driver mutations initiating development of colorectal cancer are presented in the gene for this protein. Protein APC is classified as recessive tumor suppressor. When inactivated, it stabilizes ß-catenin, which in turn leads to overexpression of genes supporting cell proliferation and progression of cancer [23].
Under physiological conditions, ß-catenin is phosphorylated by GSK and CK1 kinases and becomes visible for SCF class of ubiquitin ligases. Ubiquitination indicates ß-catenin to degradation in proteasome. However, in some cancer cell lines, mutations that eliminate target amino acids residues for these two kinases were discovered. Thus, not only mutations in the gene for APC can lead to cancer transformation. Mutations of CTNNB1 gene are associated with very poor prognosis for patients with colorectal carcinoma [24].
Thanks to the knowledge of changes involved in oncogene activation and stabilization of ß-catenin, this protein seems to be a promising target for anticancer treatment [25].
Therapy
Therapy, that targets to Wnt-ß-catenin pathway is one of the options how to prevent interaction of ß-catenin with Tcf/Lef transcription factors. This can be achieved with active form of vitamin D – 1,25-dihydroxyvitamin D3. ß-catenin can bind to the receptor of this vitamin D3, which leads to a decrease of the unbound ß-catenin level with potential to interact with Tcf/Lef. Active vitamin D3 can also increase expression of E-cadherin gene and drives ß-catenin to the protein complexes of adherent junctions so that it cannot be translocated to the nucleus [22].
Another option is the utilization of molecules such as genistein, izoflavon a fytoestrogen, that stimulate GSK3 and E-cadherin expression and lead to a decreased level of ß-catenin in cytoplasm. Such therapy in combination with other chemotherapeutics could be an important treatment for patients in metastatic phase of colorectal cancer [22].
Some small molecules can also serve as therapeutics. For example, transcription co-activator antagonist PRI-724, that increases binding of ß-catenin to p300 protein and competes with binding of ß-catenin to CBP (CRE binding protein). This prioritizes cell differentiation over proliferation. Another antagonists are for example PNU74654, 2,4-diamino-quinazolin, PKF115-584 and CGP 049090, than can also decrease interactions of ß-catenin with Tcf/Lef. There are also some molecules, that can target to other members of Wnt pathway such as Dishevelled, CK1, or Axin [22].
References
- ↑ 1.0 1.1 Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787-90. PMID:9065402
- ↑ Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998 Mar;18(3):1248-56. doi: 10.1128/MCB.18.3.1248. PMID:9488439 doi:http://dx.doi.org/10.1128/MCB.18.3.1248
- ↑ Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007 Apr 11;27(15):4210-9. doi: 10.1523/JNEUROSCI.4193-06.2007. PMID:17428999 doi:http://dx.doi.org/10.1523/JNEUROSCI.4193-06.2007
- ↑ Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of beta-catenin. Cell. 1997 Sep 5;90(5):871-82. PMID:9298899
- ↑ Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol. 1994 Dec;127(6 Pt 2):2061-9. PMID:7806582
- ↑ Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996 Aug 9;86(3):391-9. PMID:8756721
- ↑ Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014 Oct 31;346(6209):1254211. doi: 10.1126/science.1254211. PMID:25359979 doi:http://dx.doi.org/10.1126/science.1254211
- ↑ Xu W, Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci. 2007 Oct 1;120(Pt 19):3337-44. doi: 10.1242/jcs.013771. PMID:17881495 doi:http://dx.doi.org/10.1242/jcs.013771
- ↑ Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996 Sep;134(5):1271-81. doi: 10.1083/jcb.134.5.1271. PMID:8794867 doi:http://dx.doi.org/10.1083/jcb.134.5.1271
- ↑ 10.0 10.1 Zhang Y, Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J Hematol Oncol. 2020 Dec 4;13(1):165. doi: 10.1186/s13045-020-00990-3. PMID:33276800 doi:http://dx.doi.org/10.1186/s13045-020-00990-3
- ↑ Bek S, Kemler R. Protein kinase CKII regulates the interaction of beta-catenin with alpha-catenin and its protein stability. J Cell Sci. 2002 Dec 15;115(Pt 24):4743-53. doi: 10.1242/jcs.00154. PMID:12432063 doi:http://dx.doi.org/10.1242/jcs.00154
- ↑ Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003 Apr;23(7):2287-97. PMID:12640114
- ↑ Schneider SQ, Finnerty JR, Martindale MQ. Protein evolution: structure-function relationships of the oncogene beta-catenin in the evolution of multicellular animals. J Exp Zool B Mol Dev Evol. 2003 Feb 15;295(1):25-44. doi: 10.1002/jez.b.6. PMID:12548541 doi:http://dx.doi.org/10.1002/jez.b.6
- ↑ Moreno-Bueno G, Gamallo C, Perez-Gallego L, Contreras F, Palacios J. beta-catenin expression in pilomatrixomas. Relationship with beta-catenin gene mutations and comparison with beta-catenin expression in normal hair follicles. Br J Dermatol. 2001 Oct;145(4):576-81. PMID:11703283
- ↑ Fattet S, Haberler C, Legoix P, Varlet P, Lellouch-Tubiana A, Lair S, Manie E, Raquin MA, Bours D, Carpentier S, Barillot E, Grill J, Doz F, Puget S, Janoueix-Lerosey I, Delattre O. Beta-catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol. 2009 May;218(1):86-94. doi: 10.1002/path.2514. PMID:19197950 doi:http://dx.doi.org/10.1002/path.2514
- ↑ Sagae S, Kobayashi K, Nishioka Y, Sugimura M, Ishioka S, Nagata M, Terasawa K, Tokino T, Kudo R. Mutational analysis of beta-catenin gene in Japanese ovarian carcinomas: frequent mutations in endometrioid carcinomas. Jpn J Cancer Res. 1999 May;90(5):510-5. PMID:10391090
- ↑ Li N, Xu Y, Li G, Yu T, Yao RE, Wang X, Wang J. Exome sequencing identifies a de novo mutation of CTNNB1 gene in a patient mainly presented with retinal detachment, lens and vitreous opacities, microcephaly, and developmental delay: Case report and literature review. Medicine (Baltimore). 2017 May;96(20):e6914. doi: 10.1097/MD.0000000000006914. PMID:28514307 doi:http://dx.doi.org/10.1097/MD.0000000000006914
- ↑ 18.0 18.1 18.2 18.3 18.4 Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. Beta-catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10330-4. doi:, 10.1073/pnas.94.19.10330. PMID:9294210 doi:http://dx.doi.org/10.1073/pnas.94.19.10330
- ↑ Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular Mechanisms of Colon Cancer Progression and Metastasis: Recent Insights and Advancements. Int J Mol Sci. 2020 Dec 24;22(1). pii: ijms22010130. doi: 10.3390/ijms22010130. PMID:33374459 doi:http://dx.doi.org/10.3390/ijms22010130
- ↑ Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, de Braud F, Wils J. Colon cancer. Crit Rev Oncol Hematol. 2010 May;74(2):106-33. doi:, 10.1016/j.critrevonc.2010.01.010. PMID:20138539 doi:http://dx.doi.org/10.1016/j.critrevonc.2010.01.010
- ↑ doi: https://dx.doi.org/https
- ↑ 22.0 22.1 22.2 22.3 Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/beta-catenin in colon cancer. Expert Opin Ther Targets. 2014 Jun;18(6):611-5. doi:, 10.1517/14728222.2014.906580. Epub 2014 Apr 5. PMID:24702624 doi:http://dx.doi.org/10.1517/14728222.2014.906580
- ↑ doi: https://dx.doi.org/https
- ↑ Pronobis MI, Rusan NM, Peifer M. A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient betacatenin destruction. Elife. 2015 Sep 22;4:e08022. doi: 10.7554/eLife.08022. PMID:26393419 doi:http://dx.doi.org/10.7554/eLife.08022
- ↑ Nadeem A, Aung KM, Ray T, Alam A, Persson K, Pal A, Uhlin BE, Wai SN. Suppression of beta-catenin signaling in colon carcinoma cells by a bacterial protein. Int J Cancer. 2021 Jul 15;149(2):442-459. doi: 10.1002/ijc.33562. Epub 2021 Mar, 26. PMID:33720402 doi:http://dx.doi.org/10.1002/ijc.33562