User:Lori Wetmore/Sandbox 2
From Proteopedia
| |||||||||
| 1k4c, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Contents |
Background Information
Potassium, a major cation in most cells, is responsible (in addition to other cations such as sodium) for the creation of the cell membrane potential, responsible for the generation of an action potential, which is necessary for a number of cellular functions such as neurotransmission, muscle contraction, and heart function. The proper balance of potassium in the cell is maintained by potassium ion pumps in the cellular membrane. To date, there are five potassium ion channels with a resolved structure (KcsA, KirBac1.1, KirBac3.1, KvAP, MthK), with KirBac3.1 being the most recently resolved, and they are all tetramers with several conserved secondary structural elements. [1] A basic diagram of a potassium channel is illustrated by the monomeric and .
There are four basic classes of potassium channels:
- Calcium-activated potassium channels (KCa), which open in response to the presence of calcium ions or other signaling molecules.
- Inward-rectifier potassium ion channel/Inwardly rectifying potassium channels (Kir, IRK), which pass current (positive charge) more easily into the cell than out of the cell.
- Tandem pore domain potassium channels (KCNK), which are constitutively open or possess high basal activation, such as the "resting potassium channels" or "leak channels" that set the negative membrane potential of neurons. When open, they allow potassium ions to cross the membrane at a rate which is nearly as fast as their diffusion through water.
- Voltage-gated potassium channels (KcsA, KvAP), which open or close in response to changes in the membrane potential/transmembrane voltage.
Channel Structure
There are over 80 mammalian genes that encode potassium channel subunits. However, potassium channels found in bacteria are amongst the most studied of ion channels, in terms of their molecular structure. Using X-ray crystallography, [2] [3] profound insights have been gained into how potassium ions pass through these channels and why sodium ions, which are much smaller than potassium ions, do not. [4] As previously mentioned, potassium channels have a tetrameric structure in which four identical protein subunits associate to form a homotetramer, or a fourfold symmetric complex arranged around a central ion conducting pore. The polypeptide chain of bacterial potassium channels comprise 158 amino acid residues folded into two transmembrane helices, a pore helix and a cytoplasmic tail of 33 residues.[2] The subunits pack together in such a way that there is a hole in the center which forms the ion pore through the membrane. Alternatively four related but not identical protein subunits may associate to form heterotetrameric complexes with pseudo-symmetry. All potassium channel subunits have a distinctive pore-loop structure that lines the top of the pore and is responsible for potassium selective permeability (i.e., the selectivity filter). This pore-loop structure is then connected to specialized gating domains unique to each type of potassium channel.
| |||||||||
| 1bl8, resolution 3.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
The C-terminal transmembrane helix (the inner helix) faces the central pore while the N-terminal helix (the outer helix) faces the lipid membrane. The four inner helices of the molecule are tilted and kinked so that the subunits open outwards. The inner helices contain the region of the polypeptide chain between the two transmembrane helices, which is a segment of about 30 amino acid residues that contains the pore helix and loop regions which form the outer portion of the channel. It is these loop regions that together form the narrow selectivity filter that is responsible for the highly specific ion selectivity of these potassium ion channels.
A good illustration of this highly conserved structure within potassium channels can be seen in the potassium channel 1b18 from Streptomyces lividans, an integral membrane protein with sequence similarity to all known K+ channels, particularly in the pore region. X-ray analysis (data to 3.2 angstroms) reveals that the four identical subunits create an inverted cone that cradles the selectivity filter of the pore in its outer end. The narrow selectivity filter is only 12 angstroms long, whereas the remainder of the pore is wider and lined with hydrophobic amino acids. A large, water-filled cavity and helix dipoles are positioned so as to overcome electrostatic destabilization of an ion in the pore. Main chain carbonyl oxygen atoms from the K+ channel signature sequence line the selectivity filter, which is held open by structural constraints to coordinate K+ ions but not smaller Na+ ions. The selectivity filter contains two K+ ions about 7.5 angstroms apart. This configuration promotes ion conduction by exploiting electrostatic repulsive forces to overcome attractive forces between K+ ions and the selectivity filter. This basic structure allows us to visualize the physical principles underlying selective K+ conduction. [2]
Selecvitity Filter/s
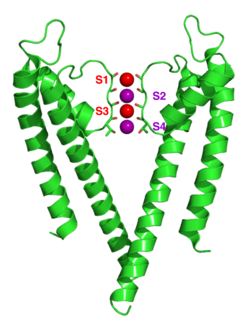
Potassium ion channels remove the hydration shell from the ion when it enters the selectivity filter, which is formed by five residues (TVGYG-in prokaryotic species) in the P loop from each subunit which have their electro-negative carbonyl oxygen atoms aligned towards the center of the filter pore and form an anti-prism similar to a water solvating shell around each potassium binding site. The distance between the carbonyl oxygens and potassium ions in the binding sites of the selectivity filter is the same as between water oxygens in the first hydration shell and a potassium ion in water solution. Passage of sodium ions would be energetically unfavorable since the strong interactions between the filter and pore helix would prevent the channel from collapsing to the smaller sodium ion size.[6] The selectivity filter opens towards the extracellular solution, exposing four carbonyl oxygens in a glycine residue (Gly79 in KcsA). The next residue towards the extracellular side of the protein is the negatively charged Asp80 (KcsA). This residue together with the five filter residues form the pore that connects the water filled cavity in the centre of the protein with the extracellular solution.[1]
The carbonyl oxygens are strongly electro-negative and cation attractive. The filter can accommodate potassium ions at 4 sites usually labelled S1 to S4 starting at the extracellular side. In addition one ion can bind in the cavity at a site called SC or one or more ions at the extracellular side at more or less well defined sites called S0 or Sext. Several different occupancies of these sites are possible. Since the X-ray structures are averages over many molecules, it is, however, not possible to deduce the actual occupancies directly from such a structure. In general, there is some disadvantage due to electrostatic repulsion to have two neighbouring sites occupied by ions. The mechanism for ion translocation in KcsA has been studied extensively by simulation techniques. A complete map of the free energies of the 24=16 states (characterised by the occupancy of the S1, S2, S3 and S4 sites) has been calculated with molecular dynamics simulations resulting in the prediction of an ion conduction mechanism in which the two doubly occupied states (S1, S3) and (S2, S4) play an essential role. The two extracellular states, Sext and S0, were found in a better resolved structure of KcsA at high potassium concentration. In free energy calculations the entire ionic pathway from the cavity, through the four filter sites out to S0 and Sext was covered in molecular dynamics(MD) simulations.[7] The amino acids sequence of the selectivity filter of potassium ion channels is conserved with the exception that an isoleucine residue in eukaryotic potassium ion channels often is substituted with a valine residue in prokaryotic channels.[1]
Channel Function
Calcium-Activated potassium channels, which include BK (big cunductance), IK (intermediate conductance), and SK (small conductance) channels, are responsible for a number of important physiological properties, including smooth muscle tone, neuronal excitability[8], electrical tuning of hair cells in the cochlea, and are also thought to be involved in synaptic plasticity, thus playing important roles in memory and learning.[9]
Inward-rectifier potassium ion channels(Inwardly rectifying potassium channels) are ubiquitously expressed and serve functions as diverse as regulation of resting membrane potential, maintenance of K(+) homeostasis, control of heart rate, and hormone secretion. [10]
Tandem pore domain potassium channels, which underlie leak K+ currents, are expressed throughout the central nervous system,[11] and currents through these channels contribute to the resting membrane potential of neurons and regulate their excitability.[12]
Voltage-gated potassium channels are sensitive to voltage changes in the cell's membrane potential and are responsoble for returning a depolarized cell to its resting state during an action potential. [13]
Gating Mechanism
A 10 Å wide central pore is located near the center of the transmembrane channel where the energy barrier is highest for the transversing ion due to the hydrophobity of the channel wall. The water-filled cavity and the polar C-terminus of the pore helices ease the energetic barrier for the ion. Repulsion by preceding multiple potassium ions is thought to aid the throughput of the ions. The presence of the cavity can be understood intuitively as one of the channel's mechanisms for overcoming the dielectric barrier, or repulsion by the low-dielectric membrane, by keeping the K+ ion in a watery, high-dielectric environment.
Ongoing Research
| |||||||||
| 1qdv, resolution 1.60Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Related: | 1qdw, 1dsx | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
One important aspect of ongoing research on potassium channels concerns determining the precise role that specific domains within the protein play in channel function. For example, all Kv voltage-gated potassium channels share a cytoplasmic assembly domain, T1. Research into whether or not this T1 domain plays a direct role in gating mechanisms has suggested that structural changes involving the buried polar T1 surfaces play a key role in the conformational changes leading to channel opening. [14] In the 1QDV, a 4 chain structure of sequences from Rattus Norvegicus (the brown rat), an isosteric mutation causes surprisingly little structural alteration while stabilizing the closed channel and increasing the stability of T1 tetramers. Replacing T1 with a tetrameric coiled-coil destabilizes the closed channel, suggesting that in mammalian Kv1.2, gating depends critically on residues at complementary T1 surfaces in an unusually polar interface.
References
- ↑ 1.0 1.1 1.2 Hellgren M, Sandberg L, Edholm O. A comparison between two prokaryotic potassium channels (KirBac1.1 and KcsA) in a molecular dynamics (MD) simulation study. Biophys Chem. 2006 Mar 1;120(1):1-9. Epub 2005 Oct 25. PMID:16253415 doi:10.1016/j.bpc.2005.10.002
- ↑ 2.0 2.1 2.2 Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998 Apr 3;280(5360):69-77. PMID:9525859
- ↑ MacKinnon R, Cohen SL, Kuo A, Lee A, Chait BT. Structural conservation in prokaryotic and eukaryotic potassium channels. Science. 1998 Apr 3;280(5360):106-9. PMID:9525854
- ↑ Armstrong C. The vision of the pore. Science. 1998 Apr 3;280(5360):56-7. PMID:9556453
- ↑ Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ Miloshevsky GV, Jordan PC. Conformational changes in the selectivity filter of the open-state KcsA channel: an energy minimization study. Biophys J. 2008 Oct;95(7):3239-51. Epub 2008 Jul 11. PMID:18621821 doi:10.1529/biophysj.108.136556
- ↑ Allen TW, Kuyucak S, Chung SH. Molecular dynamics study of the KcsA potassium channel. Biophys J. 1999 Nov;77(5):2502-16. PMID:10545352
- ↑ Wu Y, Yang Y, Ye S, Jiang Y. Structure of the gating ring from the human large-conductance Ca(2+)-gated K(+) channel. Nature. 2010 Jul 15;466(7304):393-7. Epub 2010 Jun 23. PMID:20574420 doi:10.1038/nature09252
- ↑ Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002 Dec 1;22(23):10163-71. PMID:12451117
- ↑ Allen TW, Kuyucak S, Chung SH. Molecular dynamics study of the KcsA potassium channel. Biophys J. 1999 Nov;77(5):2502-16. PMID:10545352
- ↑ Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001 Oct 1;21(19):7491-505. PMID:11567039
- ↑ Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005 Dec 7;25(49):11455-67. PMID:16339039 doi:10.1523/JNEUROSCI.3153-05.2005
- ↑ Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001 May 25;88(10):1012-9. PMID:11375270
- ↑ Minor DL, Lin YF, Mobley BC, Avelar A, Jan YN, Jan LY, Berger JM. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell. 2000 Sep 1;102(5):657-70. PMID:11007484

