User:Lori Wetmore/Sandbox 3
From Proteopedia
Contents |
ClC Channels and Transporters
Basic Function
The ClC family of chloride channels and transporters are a group of proteins that transport Cl- ions across plasma or intracellular membranes. ClC channels are unrelated in sequence to all other ion-transporting channels, including other Cl- and anion-transporting channels. ClC channels serve many functions within the prokaryotic and eukaryotic cell. Within prokaryotes, ClC channels function to help maintain cell pH, such as in the extreme acid resistance response in E. coli [1]. Within eukaryotes, and mammals specifically, ClC channels are found in many different tissue types and provide a wide variety of services such as acidifying intracellular vesicles[2], returning the resting membrane potentials of muscles to normal[3], and allowing synaptic transmission in neurons[4].
ClC channels are capable of moving ions in either direction across the membrane. However, in the majority of this article, for simplicity's sake, movement of ions will be described as if Cl- ions are moving from the extracellular→intracellular environment.
Functions within Mammals
Members of the ClC family of chloride channels are found in all of the kingdoms. Mammals contain 9 different types of ClC channels[5]. Many of the known functions of mammalian ClC channels have been determined based on the diseases caused in their absence. For example, a mutated form of the skeletal muscle ClC-1 channel in humans, mice, and goats leads to myotonia, a neuromuscular disease in which muscles have difficulty relaxing. This disease phenotype helped to implicate the ClC-1 channels in returning the resting membrane potential of skeletal muscles back to normal.
Though the basic structure of these channels is the same (further explained in later sections), there are many differences in intracellular localization, tissue residence, Cl- transport vs. Cl-/H+ antiporter function, and gating properties[6] between the various members of the ClC family. The chart below shows the members of the ClC family that are present in humans and the various characteristics of these proteins.
| ClC Channels within Homo sapiens | ||||
|---|---|---|---|---|
| Channel Name | Tissue | Location Within Cell | Basic Function | Gated By |
| ClC-1 | Skeletal Muscle | Plasma Membrane | Plasma Membrane Ion Channel | Voltage, pH |
| ClC-2 | Many (Brain, Retina, Intestine, etc.) | Plasma Membrane | Plasma Membrane Ion Channel | Voltage, Cell Swelling |
| ClC-3 | Many (Brain, Kidney, Liver, etc.) | Endosomes, Synaptic Vesicles | H+/Cl- Exchange Transporter | Voltage, Cell Swelling, Phosphorylation |
| ClC-4 | Many (Brain, Skeletal Muscle, Heart, etc.) | Intracellular Membranes (tentative) | H+/Cl- Exchange Transporter | Voltage, pH |
| ClC-5 | Kidney, Intestine, Liver | Endosomes | H+/Cl- Exchange Transporter | Voltage, pH |
| ClC-6 | Many | Endosomes | H+/Cl- Exchange Transporter | Voltage |
| ClC-7 | Many | Endosomes | H+/Cl- Exchange Transporter | Voltage |
| ClC-Ka | Kidney, Inner Ear | Plasma Membrane | Plasma Membrane Ion Channel | Voltage |
| ClC-Kb | Kidney, Inner Ear | Plasma Membrane | Plasma Membrane Ion Channel | Voltage |
The Structure of ClC Channels
|
Determining ClC Channel Structure
Though the basic types and functions of many eukaryotic ClC channels have been elucidated, no exact structural information (i.e. crystallography structures) exists for eukaryotic ClC channels. Therefore, much of the current knowledge regarding ClC channel structure has come from the elucidation of the ClC structures of prokaryotes such as E. coli[7] and S. typhimurium[8]. How much information on the function of eukaryotic channels can be drawn from the prokaryotic structure? Overall, the sequence similarity between prokaryotic and eukaryotic ClC channels is low. In addition, prokaryotic ClC channels differ from eukaryotic ClC channels in the composition of their amino and carboxy terminal domains. Eukaryotes have a larger, intracellular carboxy terminal domain that is not present within prokaryotic ClC channels[9]. These differences, however, do not eliminate the ability to study eukaryotic ClC channel function with prokaryotic models. As will be elaborated on later, several selectivity filter and gating residues are conserved amongst prokaryotes and eukaryotes [10][11][12], allowing Cl- transport within eukaryotic ClC channels to be studied within prokaryotic models.
Basic Structure
On the left is the X-ray structure of the from the bacteria S. typhimurium. The ClC channel is composed of two subunits, with each subunit consisting of 18 alpha helices. Each subunit is composed of two , that, at their interface, form the selectivity filter of the Cl- ions. The two subunits form a dimer, and there is an extensive interface between the two subunits. However, the interaction between the two dimers is not necessary for pore formation[13].Instead, the basic structure of ClC channels is that of a , in which each of the subunits contains its own pore, and two subunit monomers combine to form a double-pore channel [14].
Gating and Ion Selectivity
Fast-Gating vs. Slow-Gating
|
All studied ClC channels have been shown to be gated by voltage [15]. However, different ClC channels exhibit a great variety of responses to specific voltage changes. For example, within humans, the ClC-1 channel closes during hyperpolarization, while the ClC-2 channel opens as a result of the same change. Other ClC channels have been shown to open or close due to other factors such as pH, cell-swelling, or phosphorylation[16].
In addition, ClC channels are voltage-gated by two different mechanisms known as fast-gating and slow-gating. Within slow-gating, which takes several seconds, both pores are opened upon hyperpolarization of the membrane. Within fast-gating, on the other hand, pores react independently of each other, and react in a matter of milliseconds[17]. This fast-gating is due to a combination of Cl- movement and protonation of glutamate residue gates, and will be explained in greater detail in the following sections.
Selectivity Filter
In order to study the selectivity filter of the ClC channels, a complex was created between the from E. coli and a . The Fab antibody was attached to the extracellular surface of the Cl- channel, and was added to stabilize the ClC channel.
Each pore contains a selectivity filter that connects the intracellular and extracellular aqueous environments and through which ions travel. This selectivity filter consists of a number of side chains and main-chain amide nitrogen atoms that allow Cl-, and, in some cases, H+ ions through the channel.
The selectivity filter consists of three possible binding sites for the chloride ion. These binding sites are known as Sint, Scen, and Sext. The Sint and Sext sites are in contact with the intracellular and extracellular environments, respectively, while the Scen site is located in centrally, between the other two sites. In the image on the right, chloride ions are bound in the . Within the site, the chloride ion interacts with main-chain amide nitrogens of Gly106 and Ser107[18]. Within the site, the chloride ion interacts with the side chains of the conserved Ser107 and Tyr445 residues as well as nitrogen atoms from the main-chain amide groups of Gly149, Ile356, and Phe357[19]. Within the site, the chloride ion interacts with main-chain amide nitrogens from Gly315, Gly316, and Phe317[20]. When the pore is closed (as in this image), the Sext site is blocked by the Glu148 residue, both preventing a Cl- ion from inhabiting the Sext site as well as keeping other Cl- ions from entering the channel.
Cl- ion Movement Through the Channel
When the extracellular gate, or Glu148 residue, is deprotonated, it remains in a closed conformation and Cl- ions are incapable of entering the channel. When this residue is protonated, it swings outward, allowing a Cl- ion to bind in the Sext site [21]. The Cl- ion is then transferred to the Scen site, at which point the Glu148 residue moves back to occlude the pore entrance. The Cl- ion then moves to the the Sint site and out of the channel. By this mechanism, two Cl- ions can be within the channel when it is closed, while three Cl- ions are capable of being in the channel when it is open[22]. A mutation in which the resembles the theorized structure of the open conformation of the ClC channel. A change in Cl- ion localization during the open and closed states of the channel can be observed by comparing with the , respectively.
Cl-/H+ Transportation
|
There is some debate as to the function of various members of the ClC family. While the ClC channel from E. coli was originally assumed to be simply a Cl- channel, work by Accardi and Miller suggested that the ClC-ec1 channel was not a Cl- channel, but instead a transporter that coupled Cl- and H+ transport [23].
As it turns out, several members of the family of ClC channels are Cl-/H+ transporters rather than simple Cl- ion channels. Given the conserved structure amongst members of the ClC family, how can these different two separate functions be justified?
Ion Channel vs. Antiporter
In order to understand the mechanisms behind transport of Cl- alone as opposed to Cl- transport coupled to H+ transport, it is worth noting the differences in the gating mechanisms of ion channels versus their active transport counterparts. Ion channels generally take on a simple "open" or "closed" state, dependent on whether they are allowing or preventing ion movement through the channel, and therefore often require only one gate that may then be switched on or off. Antiporters, on the other hand, must be gated on at least one end at all times in order to properly coordinate the transport of two separate ions in different directions, and therefore often require multiple gates or gating mechanisms to regulate this transport.
It seems counterintuitive that a similar structure within all ClC channels could yield two decidedly different functions. Within ClC channels that act solely to transport Cl- ions, the conserved Glu148 residue acts as the single gate that allows or prevents Cl- flow by the mechanism mentioned above. However, the glutamate gate is only a small part of the necessary components of Cl-/H+ antiporter gating.
Coordinating Cl- Transport
While the Glu148 residue, or , ensures that extracellular ions are maintained by blocking the Sext entrance, an is created by the Ser107, Tyr445, and Phe348 residues[24], preventing ions from exiting or entering via the intracellular pore.
Coordinating H+ Transport
The Glu148 residue acts as the "end of the line" for H+ ion transport. In order for H+ ions to be transported, a residue on the intracellular side of the selectivity filter, Glu203 is protonated. The H+ ions are then transported through the channel to the Glu148 residue and into the extracellular environment.
The distance between the Glu148 and the Glu203 residues is [25], and there must, therefore, be an intermediate location for protonation. The exact pathway of H+ transport has not yet been elucidated, but it has been suggested that the residue within the Cl- selectivity filter may be involved[26].
A Model for Cl-/H+ Transport
Given what is known separately about H+ and Cl- transport within the channel, a model[27] (Figure 1) has been developed that may explain the coordination of Cl- and H+ transport within the ClC antiporter. The movement of the Cl- and H+ ions is believed to be coordinated and to occur simultaneously. As ClC channels are capable of moving ions in either direction depending on the ion gradient, this steps in this model may be reversed. However, the model will be described as if Cl- ions are being moved in an extracellular→intracellular direction, while H+ ions are being moved in an intracellular→extracellular direction. (1) Initially, both glutamate gates are deprotonated, the Glu148 residue is blocking the extracellular entrance, and a Cl- ion is present in the Scen site. (2) The intracellular gate, Glu203, is protonated by an intracellular H+ ion, which then proceeds to move to the Scen site (3) and protonate the Cl- ion. When a proton is present at the Scen site, the intracellular gate opens. (4) This opening allows Cl- to leave the channel via the intracellular exit, while the H+ ion protonates the extracellular Glu148 residue. (5) When this glutamate gate is protonated, it opens, allowing two Cl- ions to inhabit the Scen and Sext sites. The extracellular glutamate gate is then deprotonated as the H+ ion leaves, causing it to want to close. However, as a Cl- ion is blocking the exit, it is incapable of closing. (6) Therefore, the glutamate residue pushes the Cl- ions through the channel, causing one to exit the intracellular pore while the other remains in the Scent site (return to (1)). By this mechanism, the ClC transporter transports 2 Cl- ions for each proton transported[28].
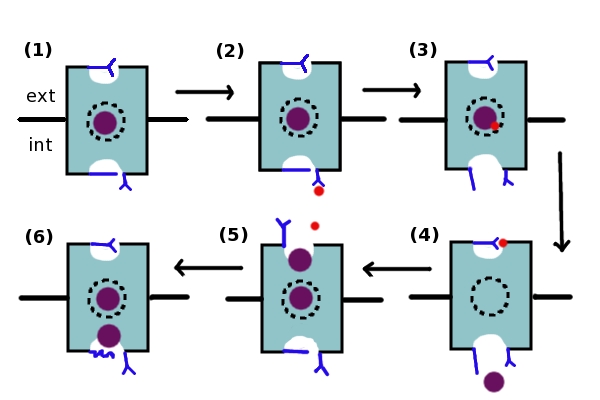
Figure 1: The coupled transport of Cl- ions and H+ ions. The blue rectangle represents one subunit of the ClC channel. The dotted circle in the middle represents the Scen site. The upper dip in the rectangle represents the extracellular pore (Sext site), while the lower dip represents the intracellular pore (Sint site). The extracellular Glu148 gate and the intracellular Glu203 residue are in blue, as is the intracellular gate composed of the Ser107, Tyr445, and Phe348 residues and represented by a blue line. The Cl- ion is represented by a purple dot, while the H+ ion is represented by a smaller red dot. The mechanism is described in detail above.
References
- ↑ Iyer R, Iverson TM, Accardi A, Miller C. A biological role for prokaryotic ClC chloride channels. Nature. 2002 Oct 17;419(6908):715-8. PMID:12384697 doi:10.1038/nature01000
- ↑ Jentsch TJ. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol. 2007 Feb 1;578(Pt 3):633-40. Epub 2006 Nov 16. PMID:17110406 doi:10.1113/jphysiol.2006.124719
- ↑ Steinmeyer K, Ortland C, Jentsch TJ. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 1991 Nov 28;354(6351):301-4. PMID:1659664 doi:http://dx.doi.org/10.1038/354301a0
- ↑ Wang XQ, Deriy LV, Foss S, Huang P, Lamb FS, Kaetzel MA, Bindokas V, Marks JD, Nelson DJ. CLC-3 channels modulate excitatory synaptic transmission in hippocampal neurons. Neuron. 2006 Oct 19;52(2):321-33. PMID:17046694 doi:10.1016/j.neuron.2006.08.035
- ↑ Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002 Apr;82(2):503-68. PMID:11917096 doi:10.1152/physrev.00029.2001
- ↑ Accardi A, Walden M, Nguitragool W, Jayaram H, Williams C, Miller C. Separate ion pathways in a Cl-/H+ exchanger. J Gen Physiol. 2005 Dec;126(6):563-70. PMID:16316975 doi:10.1085/jgp.200509417
- ↑ Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003 Apr 4;300(5616):108-12. Epub 2003 Mar 20. PMID:12649487 doi:10.1126/science.1082708
- ↑ Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002 Jan 17;415(6869):287-94. PMID:11796999 doi:10.1038/415287a
- ↑ Miller C. ClC channels: reading eukaryotic function through prokaryotic spectacles. J Gen Physiol. 2003 Aug;122(2):129-31. PMID:12885874 doi:10.1085/jgp.200308898
- ↑ Miller C. ClC chloride channels viewed through a transporter lens. Nature. 2006 Mar 23;440(7083):484-9. PMID:16554809 doi:10.1038/nature04713
- ↑ Matulef K, Maduke M. The CLC 'chloride channel' family: revelations from prokaryotes. Mol Membr Biol. 2007 Sep-Dec;24(5-6):342-50. PMID:17710638 doi:10.1080/09687680701413874
- ↑ Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004 Feb 26;427(6977):803-7. PMID:14985752 doi:10.1038/nature02314
- ↑ Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002 Jan 17;415(6869):287-94. PMID:11796999 doi:10.1038/415287a
- ↑ Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002 Apr;82(2):503-68. PMID:11917096 doi:10.1152/physrev.00029.2001
- ↑ Mindell JA, Maduke M. ClC chloride channels. Genome Biol. 2001;2(2):REVIEWS3003. Epub 2001 Feb 7. PMID:11182894
- ↑ Mindell JA, Maduke M. ClC chloride channels. Genome Biol. 2001;2(2):REVIEWS3003. Epub 2001 Feb 7. PMID:11182894
- ↑ Mindell JA, Maduke M. ClC chloride channels. Genome Biol. 2001;2(2):REVIEWS3003. Epub 2001 Feb 7. PMID:11182894
- ↑ Accardi, A. Structure and Function of CLC Chloride Channels and Transporters. Advances in Molecular and Cell Biology. 2006:56-82.
- ↑ Miller C, Nguitragool W. A provisional transport mechanism for a chloride channel-type Cl-/H+ exchanger. Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):175-80. PMID:18977737 doi:10.1098/rstb.2008.0138
- ↑ Miloshevsky GV, Jordan PC. Anion pathway and potential energy profiles along curvilinear bacterial ClC Cl- pores: electrostatic effects of charged residues. Biophys J. 2004 Feb;86(2):825-35. PMID:14747318 doi:10.1016/S0006-3495(04)74158-2
- ↑ Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature. 2002 Jan 17;415(6869):287-94. PMID:11796999 doi:10.1038/415287a
- ↑ Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003 Apr 4;300(5616):108-12. Epub 2003 Mar 20. PMID:12649487 doi:10.1126/science.1082708
- ↑ Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature. 2004 Feb 26;427(6977):803-7. PMID:14985752 doi:10.1038/nature02314
- ↑ Miloshevsky GV, Hassanein A, Jordan PC. Antiport mechanism for Cl(-)/H(+) in ClC-ec1 from normal-mode analysis. Biophys J. 2010 Mar 17;98(6):999-1008. PMID:20303857 doi:10.1016/j.bpj.2009.11.035
- ↑ Matulef K, Maduke M. The CLC 'chloride channel' family: revelations from prokaryotes. Mol Membr Biol. 2007 Sep-Dec;24(5-6):342-50. PMID:17710638 doi:10.1080/09687680701413874
- ↑ Matulef K, Maduke M. The CLC 'chloride channel' family: revelations from prokaryotes. Mol Membr Biol. 2007 Sep-Dec;24(5-6):342-50. PMID:17710638 doi:10.1080/09687680701413874
- ↑ Miller C, Nguitragool W. A provisional transport mechanism for a chloride channel-type Cl-/H+ exchanger. Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):175-80. PMID:18977737 doi:10.1098/rstb.2008.0138
- ↑ Miller C, Nguitragool W. A provisional transport mechanism for a chloride channel-type Cl-/H+ exchanger. Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):175-80. PMID:18977737 doi:10.1098/rstb.2008.0138
