This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Marion Wehrung/Sandbox
From Proteopedia
Fusion proteins (also called chimeric proteins) are proteins created by joining two genes that coded for separated proteins. Translation of this fusion gene lead to formation of new polypeptide with functional properties derived from original proteins. Alpha-synuclein fused to maltose-binding protein is a fusion protein. Alpha-synuclein is an abundant protein in human brain and to a lesser extent in muscles, heart and other tissues. It plays an important role in presynaptic terminals found in neurons and can interact with phospholipids and proteins. Studies have shown that a bad folding of this protein could lead to the formation of insoluble fibrils and thereby be a cause of Parkinson’s disease.[1] Maltose-binding protein is find in bacteria. The fusion protein allows to see the structure of alpha-synuclein and to study it by X-rays.
|
Contents |
Alpha-synuclein
Structural informations
is a 140 amino acids of about 14 kDa protein encoded by the SNCA gene.[2] It does not have a defined structure but it can form α-helical structures by binding to phospholipids for example and β-sheet structure. It is composed of three distinct regions. The first is the amino terminus (residues 1 to 60) which is lysine-rich and modulates its interactions with membranes. It contains apolipoproteins lipid-binding motifs which allow to form α-helical structures on membrane binding. It is composed of four repeated domains (20-30, 31-41, 42-56, 57-67)amino acid which look like following : [EGS]-K-T-K-[EQ]-[GQ]-V-X. [3] The central region contains a hydrophobic motif from residue 61 to 95 known as the non-amyloid-β component involved in the protein aggregation, it confers the β-sheet potential. Finally, an acidic carboxy-terminal domain, rich in proline and highly negatively charged, which is implicated in regulating its nuclear localization and interactions with small molecules, other proteins and metals and which seems to be unstructured.[4] Synucleins are small soluble proteins.
A soluble tetramer of alpha-synuclein has been identified by W. Wang research team which is the result of intersubunit interactions in central region (residues 61 to 95). Their studies suggest that the protein structure depends on subunit concentration and environmental factors. In vitro, an equilibrium between unfolded monomer, compact oligomer and amyloid-forming species can be observe. (The details of alpha-synuclein aggregation and propagation will be develop in another part.) This tetramer appears to be resistant to aggregation, following there is a model of the structure.[5]
It also exists mutation on SNCA gene that enhance the risk of catching Parkinson’s disease. The mutation of glutamic acid-46 into a lysine residue, histidine-50 into glutamin residue or alanine-53 into threonin residue increase fibrils formation and oligomerization. The phosphorylation of serin-129 also promoted the formation of unsoluble fibrils.
Protein function
Alpha-synuclein is located in the presynaptic region and is associated with the reserve pool of synaptic vesicles, so it plays a role in the regulation of neurotransmitters and synaptic function and plasticity. It is known to interact with the Rab small GTPases family and the phospholipase D2. Some studies even mention that it can act as a chaperone and affects the assembly, distribution and degradation of SNARE protein complex. This protein is highly studied because it may be the cause of Parkinson’s disease. The pathogenesis of Parkinson’s disease is probably due to the transmission cell to cell of misfolded alpha-synuclein insoluble fibrils forming aggregates named Lewy’s bodies in neurons. Since 1998 Parkinson’s disease has been classed in synucleinopathy. Lewy’s bodies are spherical cytoplasmic inclusions, situated in brain stem and composed of a dense core surrounded by fibrils of about 10 nanometers long. [6]
Alpha-synuclein has other functions. Oligomers can form pores on cellular membranes or alter the properties of voltage-gated receptors leading to excess calcium influx. This disturbs the calcium homeostasis and hence presynaptic signalling. In the same way oligomers can attack synaptic vesicles and bring out a leakage of neurotransmitters in the cytosol. [7]For example in the case of dopamine vesicles, an excess of intracellular calcium causes an augmentation of cytosolic dopamine and alpha-synuclein was required for death. This show a possible self-feeding cascade leading to neurodegeneration.
Some treatments are studied to act on alpha-synuclein protein. In 2016, the identification of lymphocyte-3 activation genes as an alpha-synuclein preformed fibrils receptors has provided a new opportunity to stop Parkinson’s disease progression. When the alpha-syn-biotine bond is misfolded to the lymphocyte-3 activation gene protein, it induces endocytosis and allows an interneuronal transmission which present a neuronal toxicity.
Moreover, it has been show that alpha-synuclein interacts with Tau2 protein. Tau protein is present in neurons and modulates the stability of the microtubules of axons by interaction with tubulin. It is mainly located in dendrites where it allows stability and flexibility of microtubules. By analysing the brain of death patient it has been prove that some areas where Tau protein presence is systematic were degenerated.
Aggregation and propagation
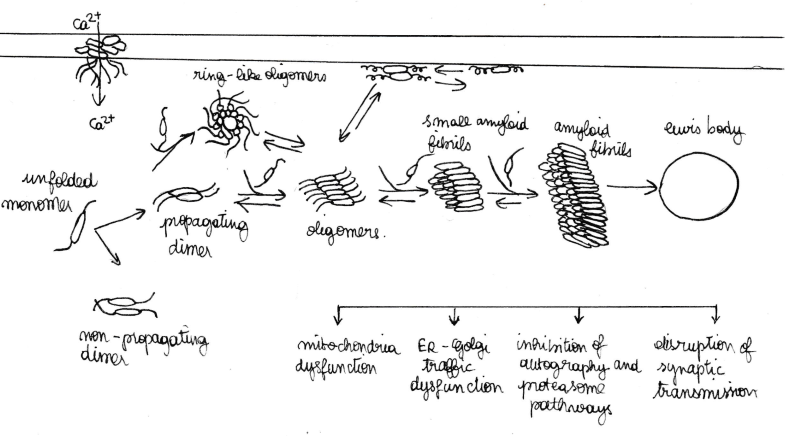
Unfolded monomers of alpha-synuclein interact and form two types of dimers: anti-parallel which do not propagate and parallel which do propagate. A dynamic equilibrium exist between unfolded monomers and the two types of dimers. Propagating dimers can grow by addition of unfolded monomers and generate oligomers and ring-link oligomers. Ring-like α-syn oligomers interact with the cytoplasmic membrane and form trans-membrane pores, inducing abnormal intracellular calcium influx. Cytoplasmic α-syn oligomers grow by the addition of soluble monomers, forming small amyloid fibrils and then longer fibrils. The accumulation of these amyloid fibrils leads to the formation of intracellular inclusions called Lewy bodies. It was marked that the most touched presynaptic buttons are those which are implicated in a dopamin synapse.[8] During α-synfibrillogenesis and aggregation, the intermediate species (oligomers and amyloid fibrils) are highly toxic, affecting mitochondrial function, endoplasmic reticulum, Golgi trafficking, protein degradation and/or synaptic transmission. The effects seen include loss of presynaptic proteins, decrease of neurotransmitter release, redistribution of SNARE proteins, enlargement of synaptic vesicles, and inhibition of synaptic vesicle recycling. These intracellular effects are thought to induce neurodegeneration. Interestingly, α-syn oligomers and fibrils, as well as the monomers, can be transferred between cells and induce disease spreading to other brain regions. Spreading mechanisms are multiple and can occur via endocytosis, direct penetration, transsynaptic transmission or via membrane receptors.
Maltose-binding protein
Structural informations
Encoded by the malE gene of Escherichia coli, Maltose-binding protein (MBP) is a monomeric protein. A deep groove which contains the maltose/matodextrins binding site separates MBP into two distinct globular domains. When MBP is liganded by maltose, a major conformational change occurs, which closes the groove.[9][10]
Protein function
MBP is involved iin the high-affinity maltose membrane transport system MalEFGK. It is also an initial receptor for the active transport of and chemotaxis toward maltooligosaccharides. In addition, MBP is a part of the maltose/maltodextrin system of Escherichia coli, which is responsible for the uptake and efficient catabolism of maltodextrins.[11]
Applications
- Increase of the solubility of fusion partner protein
Maltose-binding protein is often used in order to increase the solubility of recombinant proteins expressed in E.coli. In these cases, the protein of interest is expressed in fusion with MBP. This MBP-fusion protein prevents the aggregation of the protein of interest. However, the mechanism by which MBP increases the solubility is not well understood.
Nowadays, there are 4 models have been proposed to explain the enhancement of the solubility by some soluble proteins like MBP to their fusion partners.
In the first model, the fusion proteins form soluble micelle-like structures in which the aggregation-prone passenger proteins are sequestered on the inside, away from the solvent, while the soluble protein domains are at the outside facing the solvent. Nominé Y et al. found the evidence of the existence of this kind of structure. In the second model, the soluble protein, considered as “chaperone magnets” of its fusion partner, initiate its fusion partner into a chaperone-mediated folding pathway. Studies of Douette et al. have shown evidence that MBP and NusA fusion proteins interact with GroEL in E.coli.[12] However, how these relatively large fusion proteins could engage the GroEL/GroES chaperone apparatus in the same way like its natural substrates do remains unknown, because the chaperone cavity seems to be too small for such large fusion proteins. In the third model, the solubility-enhancing proteins possess an intrinsic chaperone-like activity that manifests itself in the context of a fusion protein. In this model, interactions between partially folded passenger proteins and hydrophobic patches on the solubility enhancer prevent their self association. Instead of playing an active role in the folding of their fusion partners, the solubility enhancers (soluble protein) might reduce unproductive off-pathway aggregation.[13] This model provides an explanation of why only certain highly soluble proteins can function as solubility enhancers. The forth model suggests that the enhancement of the solubility of fusion-protein is correlated with the net charges of the soluble protein.[14]
David S. Waugh and Sreedevi Nallamsetty found that MBP-fusion protein corresponds to the third model. In their opinion, MBP possesses intrinsic chaperone-like qualities in the context of a fusion protein, and that the hydrophobic ligand-binding cleft plays a central role in its mechanism of action.[15]
- Purification of recombinant proteins
MBP can be used as an affinity tag to purify recombinant proteins. The fusion proteins bind to amylose columns, while the non-fusion proteins flow through the column. After the acquisition of purified fusion protein, the protein of interest X can be cleaved from MBP by adding specific protease.[16]
The MBP-fusion protein usually enhances the solubility of protein X and facilitates their proper folding so that the fusion proteins are most often bifunctional.[17][18] Besides, this kind of fusions can facilitate the crystallization of difficult proteins, e.g. membrane proteins.[19]
Fused protein
To study the conversion from unfolded monomers to amyloid aggregates, scientists used maltose-binding protein to crystallize segments of alpha-synuclein. After fusion, the protein was observable with X-rays.[20]
References
- ↑ “Alpha-Synuclein.” Wikipedia, January 13, 2017. [1].
- ↑ “SNCA Gene.” Genetics Home Reference. Accessed January 25, 2017. [2].
- ↑ Okochi, Masayasu, Jochen Walter, Akihiko Koyama, Shigeo Nakajo, Minami Baba, Takeshi Iwatsubo, Laurent Meijer, Philipp J. Kahle, and Christian Haass. “Constitutive Phosphorylation of the Parkinson’s Disease Associated α-Synuclein.” Journal of Biological Chemistry 275, no. 1 (January 7, 2000): 390–97. doi:10.1074/jbc.275.1.390. Pronin, Alexey N., Andrew J. Morris, Andrei Surguchov, and Jeffrey L. Benovic. “Synucleins Are a Novel Class of Substrates for G Protein-Coupled Receptor Kinases.” Journal of Biological Chemistry 275, no. 34 (August 25, 2000): 26515–22. doi:10.1074/jbc.M003542200. Nakamura, Takeshi, Hiroshi Yamashita, Tetsuya Takahashi, and Shigenobu Nakamura. “Activated Fyn Phosphorylates α-Synuclein at Tyrosine Residue 125.” Biochemical and Biophysical Research Communications 280, no. 4 (February 2, 2001): 1085–92. doi:10.1006/bbrc.2000.4253. Fujiwara, Hideo, Masato Hasegawa, Naoshi Dohmae, Akiko Kawashima, Eliezer Masliah, Matthew S. Goldberg, Jie Shen, Koji Takio, and Takeshi Iwatsubo. “Alpha-Synuclein Is Phosphorylated in Synucleinopathy Lesions.” Nature Cell Biology 4, no. 2 (February 2002): 160–64. doi:10.1038/ncb748. Takahashi, Tetsuya, Hiroshi Yamashita, Yoshito Nagano, Takeshi Nakamura, Hiromitsu Ohmori, Hava Avraham, Shalom Avraham, Mineo Yasuda, and Masayasu Matsumoto. “Identification and Characterization of a Novel Pyk2/related Adhesion Focal Tyrosine Kinase-Associated Protein That Inhibits Alpha-Synuclein Phosphorylation.” The Journal of Biological Chemistry 278, no. 43 (October 24, 2003): 42225–33. doi:10.1074/jbc.M213217200. Khalaf, Ossama, Bruno Fauvet, Abid Oueslati, Igor Dikiy, Anne-Laure Mahul-Mellier, Francesco Simone Ruggeri, Martial K. Mbefo, et al. “The H50Q Mutation Enhances α-Synuclein Aggregation, Secretion, and Toxicity.” The Journal of Biological Chemistry 289, no. 32 (August 8, 2014): 21856–76. doi:10.1074/jbc.M114.553297.
- ↑ Lashuel, Hilal A., Cassia R. Overk, Abid Oueslati, and Eliezer Masliah. “The Many Faces of α-Synuclein: From Structure and Toxicity to Therapeutic Target.” Nature Reviews. Neuroscience 14, no. 1 (January 2013): 38–48. doi:10.1038/nrn3406.
- ↑ W, Wang, Perovic I, Chittuluru J, Kaganovich A, Nguyen Lt, Liao J, Auclair Jr, et al.“A Soluble α-Synuclein Construct Forms a Dynamic Tetramer., A Soluble α-Synuclein Construct Forms a Dynamic Tetramer.” Proceedings of the National Academy of Sciences of the United States of America, Proceedings of the National Academy of Sciences of the United States of America 108, 108, no. 43, 43 (October 25, 2011): 17797, 17797–802. doi:10.1073/pnas.1113260108, 10.1073/pnas.1113260108.
- ↑ Stefanis, Leonidas. “α-Synuclein in Parkinson’s Disease.” Cold Spring Harbor Perspectives in Medicine 2, no. 2 (February 2012). doi:10.1101/cshperspect.a009399.
- ↑ Nemani, Venu M., Wei Lu, Victoria Berge, Ken Nakamura, Bibiana Onoa, Michael K. Lee, Farrukh A. Chaudhry, Roger A. Nicoll, and Robert H. Edwards. “Increased Expression of Alpha-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering After Endocytosis.” Neuron 65, no. 1 (January 14, 2010): 66–79. doi:10.1016/j.neuron.2009.12.023.
- ↑ Maroteaux, L., J. T. Campanelli, and R. H. Scheller. “Synuclein: A Neuron-Specific Protein Localized to the Nucleus and Presynaptic Nerve Terminal.” Journal of Neuroscience 8, no. 8 (August 1, 1988): 2804–15.
- ↑ Zhao, Minglei, Duilio Cascio, Michael R. Sawaya, and David Eisenberg. “Structures of Segments of α-Synuclein Fused to Maltose-Binding Protein Suggest Intermediate States during Amyloid Formation.” Protein Science 20, no. 6 (June 1, 2011): 996–1004. doi:10.1002/pro.630.
- ↑ Spurlino, J. C., G. Y. Lu, and F. A. Quiocho. “The 2.3-A Resolution Structure of the Maltose- or Maltodextrin-Binding Protein, a Primary Receptor of Bacterial Active Transport and Chemotaxis.” Journal of Biological Chemistry 266, no. 8 (March 15, 1991): 5202–19.
- ↑ Hazelbauer, G L. “Maltose Chemoreceptor of Escherichia Coli.” Journal of Bacteriology 122, no. 1 (April 1975): 206–14.
- ↑ Douette, Pierre, Rachel Navet, Pascal Gerkens, Moreno Galleni, Daniel Lévy, and Francis E. Sluse. “Escherichia Coli Fusion Carrier Proteins Act as Solubilizing Agents for Recombinant Uncoupling Protein 1 through Interactions with GroEL.” Biochemical and Biophysical Research Communications 333, no. 3 (August 5, 2005): 686–93. doi:10.1016/j.bbrc.2005.05.164.
- ↑ Nallamsetty, Sreedevi, and David S. Waugh. “Solubility-Enhancing Proteins MBP and NusA Play a Passive Role in the Folding of Their Fusion Partners.” Protein Expression and Purification 45, no. 1 (January 2006): 175–82. doi:10.1016/j.pep.2005.06.012.
- ↑ Zhang, Yian-Biao, Jason Howitt, Sean McCorkle, Paul Lawrence, Karen Springer, and Paul Freimuth. “Protein Aggregation during Overexpression Limited by Peptide Extensions with Large Net Negative Charge.” Protein Expression and Purification 36, no. 2 (August 2004): 207–16. doi:10.1016/j.pep.2004.04.020.
- ↑ Nallamsetty, Sreedevi, and David S. Waugh. “Mutations That Alter the Equilibrium between Open and Closed Conformations of Escherichia Coli Maltose-Binding Protein Impede Its Ability to Enhance the Solubility of Passenger Proteins.” Biochemical and Biophysical Research Communications 364, no. 3 (December 21, 2007): 639–44. doi:10.1016/j.bbrc.2007.10.060.
- ↑ Nominé, Yves, Tutik Ristriani, Cécile Laurent, Jean-François Lefèvre, Étienne Weiss, and Gilles Travé. “Formation of Soluble Inclusion Bodies by HPV E6 Oncoprotein Fused to Maltose-Binding Protein.” Protein Expression and Purification 23, no. 1 (October 2001): 22–32. doi:10.1006/prep.2001.1451.
- ↑ Bedouelle, Hugues, and Pascale Duplay. “Production in Escherichia Coli and One-Step Purification of Bifunctional Hybrid Proteins Which Bind Maltose.” European Journal of Biochemistry 171, no. 3 (February 1, 1988): 541–49. doi:10.1111/j.1432-1033.1988.tb13823.x.
- ↑ Kapust, Rachel B., and David S. Waugh. “Escherichia Coli Maltose-Binding Protein Is Uncommonly Effective at Promoting the Solubility of Polypeptides to Which It Is Fused.” Protein Science 8, no. 8 (January 1, 1999): 1668–74. doi:10.1110/ps.8.8.1668.
- ↑ Waugh, David S. “Crystal Structures of MBP Fusion Proteins.” Protein Science 25, no. 3 (March 1, 2016): 559–71. doi:10.1002/pro.2863.
- ↑ Zhao, Minglei, Duilio Cascio, Michael R. Sawaya, and David Eisenberg. “Structures of Segments of α-Synuclein Fused to Maltose-Binding Protein Suggest Intermediate States during Amyloid Formation.” Protein Science 20, no. 6 (June 1, 2011): 996–1004. doi:10.1002/pro.630.