Introduction
(VKOR) is a reducing enzyme composed of 4-helices that spans the endoplasmic reticulum as a transmembrane protein[1]. Its enzymatic role is reducing (KO) to Vitamin K Hydroquinone (KH2)[2] (Figure 1). One of VKOR's primary roles is to assist in blood coagulation through this KH2 regeneration mechanism.
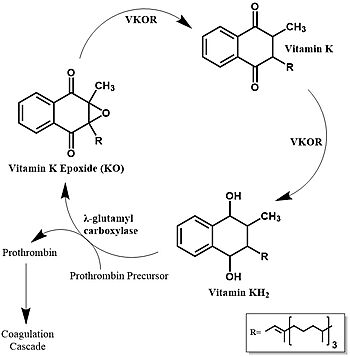
Figure 1. Vitamin K Cycle The three states of Vitamin K are shown along with important enzymes, including VKOR.
Vitamin K is essential for blood clotting in the body and is recycled by means of the Vitamin K Cycle [3] (Figure 1). The fully reduced form, KH2, is used by gamma-glutamyl carboxylase to carboxylate protein-bound glutamate residues in blood clotting cofactor precursors [4]. After carboxylation, the clotting cofactors (such as prothrombin) can bind to calcium and initiatw the coagulation cascade [5]. During this process, KH2 becomes oxidized to Vitamin K epoxide, or KO [4]. VKOR returns the epoxide to the fully reduced form for reuse by gamma-glutamyl carboxylase. This transformation happens in two steps: 1) converting the epoxide to the partially oxidized Vitamin K quinone and 2) converting the quinone to the fully reduced hydroquinone (KH2) (Figure 1) [3].
The Vitamin K Cycle and VKOR enzyme are common drug targets for thromboembolic diseases, as coagulant factor activation promotes blood clotting, which in high amounts can be dangerous and cause thromboembolic diseases such as stroke, deep vein thrombosis, and/or pulmonary embolism.
Vitamin K Epoxide Reductase is found and primarily synthesized in the liver.
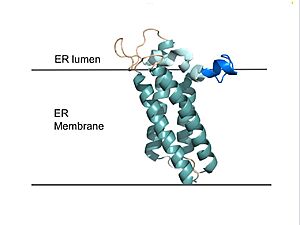
Figure 2. VKOR in Membrane Transmembrane helices are teal. The tan sections are the loops (loop 1, beta hairpin, cap loop, 3-4 loop). The cap helix is light blue and the anchor is dark blue.
Author's Note
To deduce VKOR structures, superfolder green flourescent protein (sGFP) was appended to the N and C termini of VKOR to provide added stability during crystallization. In some of the resolved structures, a catalytic cysteine was also mutated to serine to freeze VKOR in specific catalytic conformations. Also, due to stability issues, some experiments used human VKOR and others used VKOR from Takifugu rubripes. The barrel domain has been removed from all structures used in this page and the amino acids renumbered to more closely match the published numbering in the referenced article [6]. In the human VKOR (HsVKOR), the catalytic cysteines that play an intricate role in the reduction of Vitamin K epoxide are 43, 51, 132, and 135. In T. rubripes (TrVKORL), the cysteines are 52, 55, 141, and 144.
Protein Structure
Structural Overview
VKOR consists of four (, , , and ) embedded in the endoplasmic reticulum membrane (Figure 2). The transmembrane helices are located in the Endoplasmic Reticulum Luminal Region, which is the region between the ER Lumen and the Cytosol. Each of these helices come together to form a central ligand binding pocket. Helices one and two are by Loop 1 and the beta hairpin region which contains two of the active cysteines, Cys43 and Cys51; these cysteines, along with Cys132 and Cys135, are essential for the reduction of KO and structural changes for the catalytic cycle[6]. VKOR also has a consisting of a helix, loop, and anchor. The anchor stabilizes the cap domain by attaching it to the surface of the ER membrane[6]. The cap domain loop helps stabilize one of the substrate-binding amino acids, Asn80[6]. The cap domain helix is involved in stabilization of certain disulfide bonds and structural changes as part of the catalytic cycle[6]. The substrate binding pocket works in conjunction with the cap domain and the anchor to facilitate conformational transitions from the to the once a substrate binds.
Cap Domain
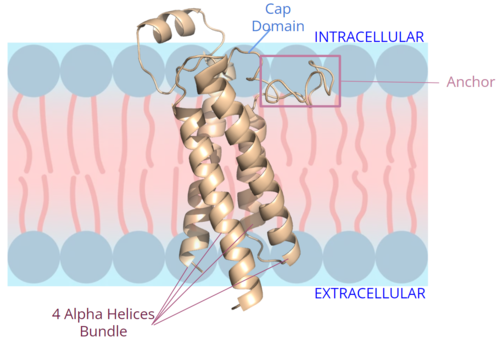
Figure 2. Orientation and interactions of the VKOR components cap domain, anchor domain, and helical bundle within the cell membrane.
The VKOR is located right above the helices of VKOR on the intracellular membrane. The cap domain has a helical shape and is located in close proximity to two other domains: the Anchor domain and beta hairpin. This combination of domains help to maintain the proper orientation in the membrane. The cap domain assists with activating Vitamin K as it induces the structural change of VKOR from the open conformation to the closed conformation upon substrate binding. Cap rearrangement and transition to the closed conformation initiates a domino effect through the catalytic mechanism[7]. The cap domain has critical interactions that stabilize the closed conformation including a between Cys43 and Cys51, and polar interactions from D44. These interactions are broken up by reactive cysteines to induce different conformations and help facilitate this transition from the open conformation to the closed conformation during the activation of Vitamin K.
Anchor
The protrudes from the side of VKOR to stabilize the enzyme within the membrane. It sits on top of the membrane and maintain proper membrane proximity for VKOR activity (Figure 2). To accomplish proper membrane orientation, hydrophilic residues on the anchor domain are positioned to interact with the outer hydrophilic leaflet of the bilipid membrane, while the hydrophobic residues on the anchor have strong interactions with the inner hydrophobic leaflet of the bilipid membrane. These control proper membrane arrangement, Vitamin K binding, and VKOR activation via cap domain movement. The anchor also stabilizes the cap domain covering the central binding pocket and keeping substrates bound in the active site.
Active Site
VKOR uses two substrate-binding amino acids, to stabilize vitamin K in the binding pocket. Tyr139 and Asn80 hydrogen bond to carbonyl groups on vitamin K and stabilize it within the binding pocket [6]. Vitamin K is also bound via hydrophobic interactions within the binding pocket of VKOR. Hydrophobic residues of VKOR, such as , form a hydrophobic tunnel within the binding pocket [6].
Catalytic Cycle
Catalytic Cysteines
The catalytic cycle of VKOR includes transitions from open to closed conformations by means of disulfide bridge-induced conformational changes (Figure 3). Open conformations (I & II) of VKOR exist when there is no ligand within the binding pocket. Closed conformations (III & IV) exist when some substrate exists within the binding pocket of VKOR. The substituent cysteines (I) act as reducing agents for the substrate, which can be either Vitamin K epoxide (KO) or partially reduced Vitamin K.
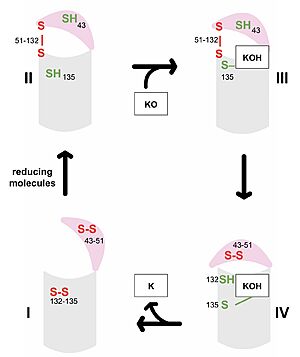
Figure 3. Catalytic Cycle of VKOR VKOR's luminal domain is represented by a the pink semicircle and the transmembrane domain is represented by the gray cylinder. Step I and II represent open conformations of VKOR and steps III and IV represent closed conformations.
The first step of the catalytic cycle (Figure 3) is the wild type open conformation. This step is characterized by an open cap domain with disulfide bonds between cysteines 43 and 51 and between cysteines 132 and 135 [6]. The second step of the catalytic cycle is a partially oxidized open conformation. This step is characterized by a disulfide bond between the luminal and transmembrane domain (Fig 3, step II). The transmembrane domain contains a free Cys135 and the luminal domain contains a free Cys43 [6]. Step II is labeled as open because no ligand exists within its binding pocket despite the disulfide bridge that connects the luminal and transmembrane domains. The next step of the cycle is a closed structure with an intact disulfide bond between Cys51 and Cys132. Cys135 is not involved in a disulfide bridge and instead reacts with substrate by forming a stable adduct with KOH or K. This binding induces the closed conformation and uses Cys43 in the luminal membrane for electron transfer [6]. The final step of the catalytic cycle is the last closed conformation. The Cys51-Cys132 bond is broken as Cys43 bonds with Cys51, recreating the disulfide bridge pattern of the open state. Cys132 is then free to bond with Cys135, releasing the product that was bound to the Cys135. Overall the catalytic cycle of VKOR is dependent on open and closed conformational changes of the protein and ultimately is used to generate vitamin K from vitamin K epoxide [6].
Medical Relevance
Warfarin
Warfarin is the most widely prescribed oral anticoagulant and targets blood clotting via inhibition of VKOR. The FDA approves the use of Warfarin for cardiac conditions (myocardial infarction, atrial fibrillation) as well as for deep vein thrombosis and pulmonary embolism. Due to the inhibition of the normal blood clotting cycle, patients taking warfarin are at risk for hemorrhage which can occur anywhere in the body. [8]
Warfarin is a of Vitamin K that occupies the VKOR binding site, acting as a competitive inhibitor. Warfarin mimics vitamin K by binding to the same (Asn80 and Tyr139) in the active site. Warfarin also shares the same within the binding site that KOH experiences (Phe83, Phe87, and Tyr88). Warfarin binding also depends on the VKOR catalytic cysteines. Warfarin is able to bind to the fully oxidized open form of VKOR as shown in of the catalytic cycle. Once Warfarin binds, VKOR is considered to be in a closed conformation since the substrate cannot enter, despite the lack of disulfide bridge changes. Warfarin can also bind to the partially oxidized form of VKOR as shown in of the catalytic cycle.
There are around 30 known missense mutations that lead to warfarin resistance in patients, but these mutations do not affect Vitamin K binding for reasons which are not yet fully understood. Such patients require higher doses of warfarin to reach therapeutic level or require a different anticoagulant drug. [9]
Superwarfarins
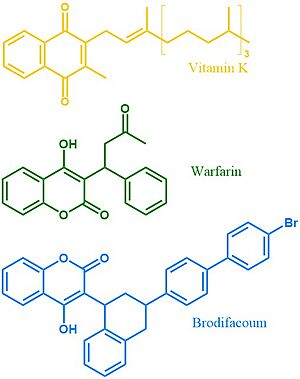
Figure 4. Vitamin K, Warfarin, and Brodifacoum Above is a comparison of the 2D structures of VKOR's natural substrate, the blood thinner warfarin, and the superwarfarin brodifacoum.
More potent warfarin derivatives, called superwarfarins, are used as rodenticides. The duration of one superwarfarin, brodifacoum, has been reported as 15-30 days [10] vs. the clinical warfarin duration of 2-5 days[8]. Superwarfarins have bulkier side chains that allow them to stay bound to VKOR for long periods of time, causing prolonged and uncontrolled bleeding[10]. The is due to interactions between the bulky side chains and the hydrophobic residues of VKOR's binding pocket, as shown in brodifacoum.





