Introduction
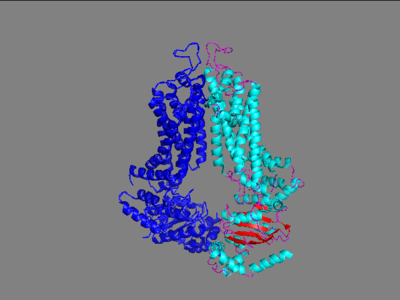
The ABCG2 transporter protein is a notable transmembrane protein that transports xenobiotic material out of numerous cells especially those located on the blood-brain barrier. ABCG2 belongs to the family of 48 transporter proteins called ATP-binding cassette transporters (ABC transporters) that are made unique from each other by their size, structure and their ordering of specific domains. This family has been found as a prevalent piece of multi-drug resistant cancers and therefore became a popular target towards inhibition. Three generations of drugs were made in order to inhibit a similar protein from the same family, ABCC1 at its interior binding site including cyclosporine A (first generation), valspodar (second generation), and Elacridar (3rd generation). Importantly, cyclosporine A and Elacridar were found to inhibit both ABCC1 and ABCG2 and in one trial had success along with chemotherapy in the treatment of acute myeloid leukemia but because of either side effects or experimentation that was not able to be duplicated, this research was mostly shelved. The main issue in their failure to find a drug to inhibit this protein was the failure to develop a high-resolution structure of this protein with the technology available at the time of this drug development. In the mid 2010's, upgrades to cryo-electron microscopy and the use of 2 antigen binding fragments (5D3-Fab) allowed for high resolution images to finally be developed for ABCG2 transporter protein. With these recent discoveries, the understanding of this protein has greatly increased in the last several years.
General Structure
The ABCG2 protein is comprised of a homodimer which each have two specific domains: one spanning the cell membrane and one involved with nucleotide binding.
Transmembrane Domains
In the transmembrane domain is located the leucine plug that separates the first site of binding from the second site of binding for the substrate. This is only opened when the protein is shifted from its interior facing to exterior facing formation by the transfer of a phosphate off of an ATP. This causes a shift and transfer to the second binding site which is open to the extracellular matrix. The Fab-FD3 antigen binding fragment was found to stabilize the protein in its inward facing conformation which allowed for high resolution images of the protein to be taken in this conformation. Without Fab attached, the conformation of the protein was constantly in flux, transporting substrates out of the cell constantly. Fab was found to only stabilize ABCG2 on the extracellular side. Three important interactions between Fab fragments and ABCG2 were important to binding stability and favorability. This binding occurred at the EL-3 of ABCG2 which is the helices that stretches the furthest from the cell membrane. These interactions were two disulfide bonds, one intramolecularly and the other intermolecularly, and an n-glycosylation site at residue Asn-596.
Nucleotide Binding Domains
These two domains contain the active site of this transporter protein. The interest in this protein is in its involvement with mutidrug resistant cancer cells. This involvement is due to its active site's promiscuity as many xenobiotics have been found to transported to the outside of the cell by this transporter.
Function
Disease
Relevance
Structural Highlights