User:Sandip Suresh/Sandbox 1
From Proteopedia
Contents |
Voltage-gated potassium channel
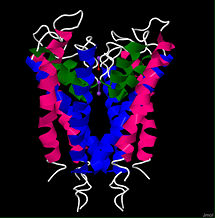
Background
Voltage-gated potassium (Kv) channels fall under a large family of closely related transmembrane proteins that are present in nearly all classes of living organisms [1][2]. Among other purposes, voltage gated potassium channels play a critical role during the repolarization phase of neuronal action potentials [3][4]. The action potential is a short-lived spike in membrane voltage that begins with a rush of Na+ ions into the neuron that subsequently causes an increase in the voltage across the plasma membrane. At the peak of the action potential, the Kv channel slowly opens up to let a K+ current flow down its gradient and out of the cell[3][5]. This crucial function of the Kv channel allows the neuron to reestablish its resting voltage and get ready for its next action potential [4][1].
Even before the structural determination of the membrane protein was possible, the physiological functions of potassium channels were studied at great lengths. Early research depended on a mutant strain of Drosophila dubbed “Shaker”, initially noticed for its eponymous behavior. When the mutant gene was cloned and sequenced, the gene was found to be similar to known ion channels[6]. Further study with channel antagonists helped define the protein’s physiological role. Tetraethylammonium was one such channel antagonist that was used to show that the channel acts as a pore that selectively allows potassium ions to cross the otherwise impermeable plasma membrane [4]. Furthermore, it was shown that the Kv channel was able to control the flow of K+ in response to changes in voltage across the membrane[3]. The astounding selectivity in choosing potassium ions to let through the membrane, as well as its ability to sense and react to the voltage across the cell’s membrane was, for many years, a fascinating enigma that accordingly garnered widespread interest by many [7] [8]. Recently, the groundbreaking structural determination of the Kv channel has begun to reveal the mechanisms by which the Kv channel manages these remarkable tasks.
| |||||||||
| 1bl8, resolution 3.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Structure
In 1998, Doyle et al.[7] were able to successfully use x-ray crystallography to determine the structure of the bacterial potassium channel from Streptomyces lividans (KcsA channel) with a resolution of 3.2 angstroms. The general structure of the potassium channel was found to consist of a complex of four identical subunits forming a pore to allow ion conductance, and a gate with a voltage sensor to regulate conductance[3]. Each subunit of the KcsA channel is 158 residues long and consists of two transmembrane helices, an which faces the center of the pore and an that faces the lipid membrane[7][9]. Each subunit also contains a near the extracellular side of the protein that links the inner and outer helix segments. As its name implies, the pore helices contribute to the pore of the channel but also provide the sites of interaction between the subunits that allow them to complex to form a stable tetramer[7].
Ion Selectivity
One of the wonders of the potassium channel is its ability to allow the larger K+ ion (1.33Å radius) to pass through the channel, yet simultaneously exclude the smaller Na+ ion (0.95 Å) from entering[7]. Interestingly, the channel is 10,000 times more selective for potassium than sodium, yet it shows very little selectivity discrimination between potassium and the next largest alkali metal, rubidium (1.65 Å)[7]. This preference for the larger monovalent cations is mediated by a that sits at the mouth of the extracellular side of the channel[7]. The highly specialized selectivity filter is fully conserved in all known potassium channels, and has a sequence of TVGYG[10][7]. The entire filter is formed when the main-chain of the conserved residues from the four subunits point towards the pore to coordinate with the potassium ion[10]. This selectivity filter creates a choke point that provides a very narrow opening to the cavity of the channel [11][7]. In a seemingly counterintuitive fashion, this choke point actually favors the larger monovalent cations over the smaller ones. The structural determination of the potassium channel showed that this is possible because the carbonyls are perfectly aligned to coordinate with the potassium ion, but the sodium ion is too small to efficiently coordinate with all of the carbonyls from each of the four subunits. The protein-ion coordination allows the selectivity filter to peel away the water molecules that form the tight hydration shell around the potassium ion, effectively reducing the size of the previously solvated cation[10][7].
The potassium channel has been measured to allow a current of 10^8 ions/second, meaning the selectivity filter is able to dehydrate a potassium ion in as little as 10 nanoseconds[10]. However, the dehydration of the potassium ion leaves it bound to the carbonyls of the selectivity filter and should, in theory, prevent it from so easily escaping the clutches of the tight coordination to the protein. So what then facilitates such an efficient throughput? From the arrangement of the carbonyls in the resolved structure, it is thought that the selectivity filter is able to bind two ions concomitantly and the electrostatic repulsion between the two adjacent positively charged ions displaces one and pushes it through the channel[10][3].
Channel cavity
Before a potassium ion enters the selectivity filter, it passes through an inner pore on the intracellular side of the protein and enters a large, water-filled cavity at the bilayer center[11][7][10]. This cavity is lined with residues, but holds approximately 50 water molecules[7][11]. The hydrophobic residues prevent strong binding interaction between the ion and the protein while the water inside the cavity is still able to stabilize the cation as it travels through the channel[7]. The C-termini of the four pore helices are angled toward the center of the cavity to assist with the electrostatic stabilization of the ion[7][3]. Each pore helix is a partial dipole, with the C-terminus being slightly negatively charged[7]. The slight negative charge points toward the K+ ion while it is in the cavity and confers selectivity of cations over anions[9].
Voltage gating
The second remarkable aspect of the voltage gated potassium channels is their ability to regulate potassium conductance by sensing and reacting to changes in membrane voltage [8]. The dependence of potassium channel gating on membrane voltage was first noted in the 1950’s by Hodgkin and Huxley[12] . They also found that as the channel moved from a low conductance to a high conductance state, a small “gating current” could be measured[12]. It was initially predicted that this gating current was the result of charged amino acid residues moving through the membrane due to a conformational change in the protein [13]. Under the assumption that the movement of these charges was responsible for the regulation of conductance, mutagenesis experiments were conducted to pinpoint which ionic residues were responsible for gating. Cordero-Morales et al.[13] found that mutation of essentially eliminated the voltage dependence of potassium conductance. A look at the structure of KcsA indicates that this residue is nestled alongside the selectivity filter. The nearby residue coordinates with Glu71 to create a carboxyl-carboxylate interaction[13]. It is thought that this interaction holds the important residues of the selectivity filter in place. As the voltage changes, this interaction could be disrupted, causing a conformational change in the residues of the selectivity filter. Once the residues of the selectivity filter are misaligned, the protein no longer allows ions to pass through[13].
Channel Homology
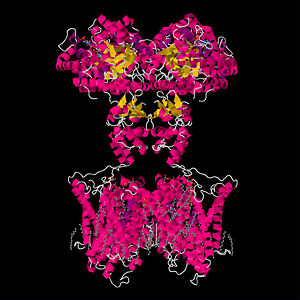
Although the structural determination of the bacterial KcsA channel was no achievement to scoff at, the crown jewel of membrane-protein crystallization was the successful determination of the staggeringly complex Shaker-related mammalian Kv channel[14]. As opposed to the elegant simplicity of the two-transmembrane helix architecture of the KcsA channel, the Shaker-related potassium channels display a daunting structural complexity that had hindered efforts to crystallize the protein[15] . Earning the Nobel prize in chemistry, Roderick MacKinnon’s lab used a mixture of lipids and detergents to successfully extract and crystallize the Kv channel from a rat in 2003[14][1].
The KcsA channel shares significant sequence and structure homology with the Shaker family of mammalian Kv channels[7]. However, each subunit of the Shaker channel consists of 6 helical transmembrane domains (S1-S6) instead of just the two of the KcsA channel[7]. The S1-S4 helices are voltage sensing domains and S5-S6 are respectively homologous to the outer and inner helices of the KcsA channel[3]. The selectivity filter structure is strongly conserved between the two proteins, and is thought to work by the same mechanism as well[5]. However, the mechanism of voltage gating in the Shaker related channels is known to occur through a much more complicated mechanism, yet is still not completely understood[15]. For many years it was thought that the S4 helix was located near the core of the protein and oriented parallel to lipids in the membrane. The gating mechanism was thought to involve the S4 helix sliding in and out of the plane of the membrane to somehow cause conformational changes in the rest of the protein[15]; [8]. However, the crystal structure of the channel indicates that the S4 helix is actually oriented perpendicularly to the bilayer, leaving it in a lipid environment[15][8]. The S4 helix is known to contain many positively charged residues, meaning it would be energetically unfavorable for the highly polarized helix to exist in the hydrophobic environment that the crystal structure indicates [15][8]. There remains a highly contentious debate over the merits of the crystal structure and the mechanism of voltage gating in Shaker related potassium channels[15][8].
References
- ↑ 1.0 1.1 1.2 Long, S. B.; Campbell, E. B.; MacKinnon, R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science 2005, 309, 897-903.
- ↑ Rasband, M. N. Clustered K+ channel complexes in axons. Neurosci. Lett. 2010, 486, 101-106.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Yellen, G. The voltage-gated potassium channels and their relatives. Nature 2002, 419, 35-42.
- ↑ 4.0 4.1 4.2 Armstrong, C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 1971, 58, 413-437.
- ↑ 5.0 5.1 Long, S. B.; Tao, X.; Campbell, E. B.; MacKinnon, R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 2007, 450, 376-382.
- ↑ Tempel, B. L.; Jan, Y. N.; Jan, L. Y. Cloning of a probable potassium channel gene from mouse brain.Nature 1988, 332, 837-839.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 Doyle, D. A.; Morais Cabral, J.; Pfuetzner, R. A.; Kuo, A.; Gulbis, J. M.; Cohen, S. L.; Chait, B. T.; MacKinnon, R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 1998, 280, 69-77.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Ahern, C. A.; Horn, R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 2004, 27, 303-307.
- ↑ 9.0 9.1 Guidoni, L.; Torre, V.; Carloni, P. Potassium and sodium binding to the outer mouth of the K+ channel.Biochemistry 1999, 38, 8599-8604.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Morais-Cabral, J.; Zhou, Y.; MacKinnon, R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature 2001, 414, 37-42.
- ↑ 11.0 11.1 11.2 Roux, B.; MacKinnon, R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science 1999, 285, 100-102.
- ↑ 12.0 12.1 Hodgkin, A. L.; Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500-544.
- ↑ 13.0 13.1 13.2 13.3 Cordero-Morales, J.; Cuello, L. G.; Perozo, E. Voltage-dependent gating at the KcsA selectivity filter. Nat Struct Mol Biol 2006, 13, 319-322.
- ↑ 14.0 14.1 Miller, G. Gateways Into Cells Usher in Nobels. Science 2003a, 302, 383-384.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Miller, G. The Puzzling Portrait of a Pore. Science 2003b, 300, 2020-2022.

